| Chapter 13: Spectroscopy |
| Chapter 13: Spectroscopy |
Interpretting 1H-NMR Spectra
Let's summarise what can be obtained from a 1H
NMR spectrum:
| How many types of H ? | Indicated by how many groups of signals there are in the spectra |
| What types of H ? | Indicated by the chemical shift of each group |
| How many H of each type are there? | Indicated by the integration (relative area) of the signal for each group. |
| What is the connectivity ? | Look at the coupling patterns. This tells you what is next to each group |
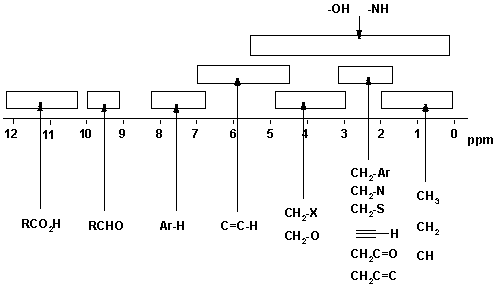
Note that the figure shows the typical chemical shifts for protons being influenced by a single group. In cases where a proton is influenced by more than one group, the effects are essentially cumulative.
An example of an H NMR is shown below.
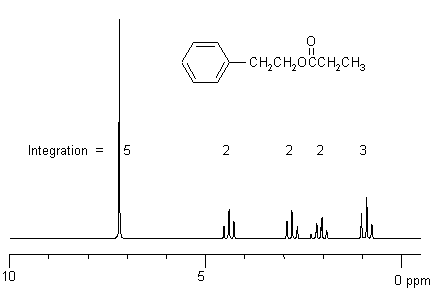
Based on the outline given above the four sets of information we get are:
5 basic types of H present in the ratio of 5 :
2 : 2 : 2 : 3.
These are seen as a 5H "singlet" (ArH), two 2H triplets, a 2H quartet and a
3H triplet. Each triplet tells us that there are 2H in the adjacent position,
and a quartet tells us that there are 3H adjacent.
(Think of it as the lines you see, L = n + 1, where n = number of equivalent
adjacent H)
This tells us we that the peaks at 4.4 and 2.8 ppm must be connected as a CH2CH2
unit.
The peaks at 2.1 and 0.9 ppm as a CH2CH3 unit. Using the
chemical shift charts, the H can be assigned to the peaks as below:
7.2ppm (5H) = ArH
4.4ppm (2H) = CH2O
2.8ppm (2H) = Ar-CH2
2.1ppm (2H) = O=CCH2CH3 and
0.9ppm (3H) = CH2CH3
| © Dr. Ian Hunt, Department of Chemistry |