| Chapter 20: Carboxylic Acid Derivatives. NucleophilicAcyl Substitution |
| Chapter 20: Carboxylic Acid Derivatives. NucleophilicAcyl Substitution |
Hydrolysis of Nitriles
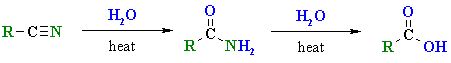
Summary
| MECHANISM
OF THE ACID CATALYSED HYDROLYSIS OF NITRILES |
|
| Step 1: An acid/base reaction. Since we only have a weak nucleophile so activate the nitrile, protonation makes it more electrophilic. |
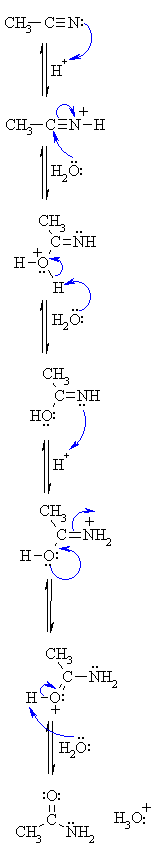 |
| Step 2: The water O functions as the nucleophile attacking the electrophilic C in the C≡N, with the electrons moving towards the positive center. |
|
| Step 3: An acid/base reaction. Deprotonate the oxygen that came from the watermolecule. The remaining task is a tautomerisation at N and O centers. |
|
| Step 4: An acid/base reaction. Protonate the N gives us the -NH2 we need.... |
|
| Step 5: Use the electrons of an adjacent O to neutralise the positive at the N and form the π bond in the C=O. |
|
| Step 6: An acid/base reaction. Deprotonation of the oxonium ion reveals the carbonyl in the amide intermediate....halfway to the acid..... What about the rest ? |
|
| © Dr. Ian Hunt, Department of Chemistry |