Here is an post-mortem analysis / "how
to" for the FINAL. The
questions
are split by the sections. At the start of each section are a few
suggestions
of what to look for or how to tackle the question type.
Qu1: D
Acidity
and basicity and / or pKas. There are two oxygen systems
and an nitrogen in an amine. Basicity can be assessed by thinking
about electron availability..... more available = easier to donate =
stronger base. Let's deal with the two oxygens first. The
negative oxygen is more basic due to the extra electron density.
Now compare the O and the N in i
and iii. Since N is less
electronegative than O, N is a better electron donor than O (pKas :
amine to ammonium about 10, alcohol to oxonium (like H3O+) is about
-2). So now we need to compare the N in ii with the O- in iii. Since a carboxylic acid
(pKa = 5) will protonate an amine (pKa about 10), we know that the
carboxylate is the weaker base. Hence ii > iii > i.
Qu2: D
Carbocation stability....for simple alkyl cations, more alkyl
groups means a more stable carbocation. i is a vinyl cation which is very
unstable, typically between primary and methyl cations. ii is a tertiary and iii is secondary, so overall we
have in terms of stability, ii
> iii > i.
Qu3: A
IR
stretching frequencies are predicted by Hooke's
Law. Here the
atoms involved are CO bonds so it's the strength of these bonds that is
the critical factor. i is a
C=O, whereas ii and iii are C-O. However the
difference in ii and iii is that is ii the C is sp2 and in iii the C is sp3. Therefore the C-O
bond in ii is stronger than
that in iii since the greater
the s character in the C hybrid, the shorter and the stronger the bond.
Hence in terms of bond strength and therefore IR stretching frequency, i > ii > iii.
Qu4: D
Alcohols react with sulfuric acid in an elimination reaction
that is SN1 in character and the reactivity is dictated by the relative
stability of the carbocations so tertiary react faster than secondary
than primary... therefore ii
> iii > i.
Qu5: C
Each type of carbon will give a single peak so it's counting types of
carbon. i has 4 types since the
system is symmetrical with 2 types of aromatic C and 2 types of sp3 C (CH2 and
CH3). ii has 5 types, no symmetry here, all the C
are different. iii has 3 types since
the molecule can be divided in half at the middle of the C=C to give 1 sp2 C
type, then 2 different CH2 groups, since one is connect to an sp2 C whereas
the other is connected to 2 sp3 C atoms. Hence
ii > i > iii.
Qu6: C
Basicity of nitrogen systems. Basicity can be assessed by thinking
about electron availability..... more available = easier to donate = stronger
base. Look at the N lone pairs.... i is an amide, the N lone pair is involved
in resonance
with the C=O. ii is an amide and there
is no resonance
delocalisation. So the amine ii
is more basic than the amide i. Finally,
iii is a nitro group... the formal
charge on the N is +ve so there is no lone pair and the N is not basic.
Hence ii > i > iii.
Qu7: B
H-nmr
chemical shifts in this system (all sp3) will be mainly affected by
electronegativity
of substituents as this will affect the shielding. First compare i and iii, both have -Br attached, the
difference is the -O. This means i
is more deshielded than iii and
so the chemical shift of i >
iii. Now compare ii and iii. ii is more remote from any of the
electronegative groups. Since the effect dies off with distance, the
chemical shift of iii > ii. Hence i
> iii > ii
Qu8: AB
All about the nucleophilicity
of these groups in SN2 reactions with the secondary alkyl
bromide. If we compare i
and ii first as both are oxygen systems.... i has charge delocalisation due to
resonance and this makes it a poorer electron donor, hence a poorer Nu
than ii. Now compare ii and iii, iodide. Since nucleophilicity
increases as you go down a group (due to size and polarisability),
iodide is a very good nucleophile, so overall we have iii
> ii > i
Qu9: D
Leaving
group ability.....a good leaving group is the conjugate base of a
strong acid (i.e.
stable is the displaced form). So we can look at the
corresponding acids, NH3, HCl and H2O respectively. HCl is the
recognisable acid here (pKa = -7) so chloride, Cl-, is a good leaving
group. Now consider the N vs O systems. H2O is a stronger acid than NH3
- yes, HO- is a poor LG, but -NH2 is even worse (since N is less
electronegative than O). So ii
> iii > i.
Qu10: D
A very similar question to the MT, application of
acidity and basicity to a simple amino acid. But not a
trivial question. The carboxylic acid (typical pKa = 5) will
protonate the amine (typical pKa about 10) so the amino acid exists as
the zwitterion in aqueous solution = (NH3+)-C6H4-CO2-
(i.e. ii). When 0.25 mole equivalent of NaOH
(a base, pKa about 15) is added to this, only about 25% of the ammonium group
will be
deprotonated to give iii
(since the pKa for the NaOH / H2O equilibrium is less
acidic than that of the ammonium ion). So the major species in
solution will still be ii.
i is not "possible"
since
the carboxylic acid is a strong enough acid to protonate the basic
amine
(look at the pKas). So ii
> iii > i
Qu11: B
Elimination
of alkyl halides is via the E2
pathway, where the H
is always removed from the C adjacent to the C-X bond. First challenge,
draw the structures (nomenclature
!)

i
can give 3 alkene products,
1-pentene and cis- and trans-2-pentene. ii can
eliminate to give only cyclopentene and iii can
eliminate to give 2 alkenes.... 2-methyl-1-butene, 2-methyl-2-butene.
Hence i > iii > ii.
Qu12: B
Really about alkene stability. Notice they are isomeric.
The more stable the alkene the more exothermic it's heat
of formation. In terms of alkene
stability, the general rule is the more alkyl groups on the C=C
unit, the more stable it is. i
has 1 alkyl groups, ii and iii both have 2, so i is the least stable and has the
least exothermic heat of formation. For non-cyclic systems, trans
isomers tend to be more stable than cis isomers, so ii is more stable than iii. Hence, in terms of the
heats of formation i > iii
> ii.
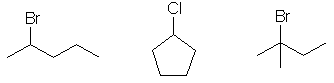
Qu 13: E
(SN1/SN2 expt) The reaction conditions are for SN1, asks for the
most reactive. The system giving the most stable carbocation will react
fastest. That will be E
since it leads to a cation that is resonance stabilised to a tertiary
carbocation. A gives
primary with resonance stabilisation, B
and C give
primary, D gives a vinyl
cation.
Qu14: D
(SN1/SN2 expt) The reaction conditions are for SN2, asks for the
least reactive. The
vinyl system D (it's an sp2
C-Br) will not react under these conditions (SN1 and SN2 are on sp3
systems).
Qu15: CD
(models & spectroscopy expts). First challenge, draw the
structures (nomenclature
!) then calculate
the IHD (1= pi, 1 = ring, 2 = 2 pi, 2 = pi + ring, 1 = pi)
Qu16: BD
(Extraction of caffeine from tea expt). A is false, caffeine is
a polar organic molecule and is more soluble in polar organic solvents such
as chloroform and dichloromethane than water. C is false... caffeine
is quite polar and is therefore not very soluble in a nonpolar solvent like
pet. ether. E the cold finger
is part of the sublimation apparatus.
Qu17: BD
(Hydrocarbon & spectroscopy expts). First, you need to draw the product
from the bromination (either reaction via the most stable radical for B
and E or via addition to the alkenes A C and D). To give
a single peak in the H-nmr, the product can only have one type of H....the radical
substitution product of E has 4 types
of H. Addition of bromine to alkenes A and C gives products with
2 types of H.
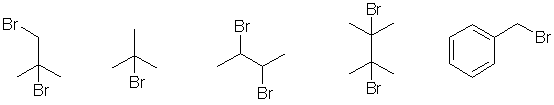
Qu18: A
The compounds are all carbonyl compounds and all the H atoms are
attached to C atoms. Therefore, in each case, the most acidic H in each
molecule will be on the C adjacent to the carbonyl groups since that
allows for resonance stabilisation in the conjugate base.
In A there is more
resonance delocalisation because the central CH2 group is adjacent to
two carbonyl groups.
Qu20: A
Propenyl acetate (or propenyl ethanoate) is CH3COOCH=CHCH3
or C5H8O2. Constitutional
isomers have the same molecular formula and different connectivity.
Qu21: BC
Identical structures will have the same melting point.
Enantiomers will also have the same melting point while diastereomers
have different physical properties. B
and C are enantiomers. D is a diastereomer of B and C. A is a constitutional isomer of the
other four. E is a
constitutional isomer of the other four
Qu22: A
A chirality center occurs when an sp3 C has four different groups
attached. The only compound here without a chirality center is A (note it's mirror plane).
Qu23: E
The IHD
here is 6 (2 C=O pi bonds, 2 C=C pi bonds and 2 rings).
Qu24: D
There are 4 types of H here.... 3 different methyl groups and
the H required at C8.
Qu25:AB
All three atoms are sp2 hybridised.
C1 is part of an alkene and has three groups attached (C2, C6 and N7).
N3 is part of an amide - there are four groups (C2, C4, C13 and the
lone pair) but the lone pair is involved in resonance with the carbonyl
group and hence is in a p orbital. O10 is part of a carbonyl
group with three groups attached (C6 and two lone pairs).
Qu 26: D
Oxidation states are calculated by looking at the distribution of the
electrons in the bonds due to electronegativity. N3 is attached to 3 C
atoms (less electronegative = 3 x +1 = +3 therefore N3 = -3) and
C6 is attached to 1 x C, 1 x N and 2 x O ( 0 + (-1) + (-2)) = -3
therefore +3.
Qu 27: E
Each type of C with give an individual signal - there are 8
types of C here (each C is unique).
Qu28: C
(1) is an SN2
of an alkyl halide to make an alkyl iodide then (2) will substitute
the new iodide to create an alcohol which then has undergone an
intramolecular Williamson type reaction to give a cyclic ether. So we
need a system with 5 C atoms and 2 different good leaving groups.
That's C. E doesn't have enough C atoms. In D the F is not a good leaving group.
Qu29: C
(1) is the radical
halogenation of an sp3 C atom (look for the most stable radical)
then (2) in an SN2 of the bromide with the very good HS-
nucleophile. A has too
many C atoms, B and D have the wrong substituent on the
aromatic ring (note aromatic -OH and -Br don't undergo simple SN1 or
SN2 reactions). C will react
with the bromine to add the Br adjacent to the benzene ring (via a
resonance stabilised radical) and then the HS- replaces the Br.
Qu30: C
(1) is alcohol
to tosylate then (2) is a nucleophilic
substitution (SN2) of the tosylate with the nucleophilic acetylide
- this will occur with inversion of stereochemistry. A has the wrong stereochemistry, B has the structure based on the
nucleophile used, D has one
too many C atoms based on the nucleophile used and E has the wrong stereochemistry, and
has one too many C atoms based on the nucleophile used.
Qu31: B
The reaction conditions are the elimination
of an alkyl halide to give an alkene. Therefore it can only be A-C. Alcohols
(D-E) don't eliminate under
basic reaction conditions. Recall that these eliminations are
1,2-eliminations
so the H and the LG leave from adjacent C atoms. therefore C would give a different alkene.
Due to the anti
requirement of the E2
reaction, A would be
forced to undergo an anti-Zaitsev elimination to give
3-methylcyclohexene rather than the 1-methylcyclohexene required.
Qu32: B
Alcohols
undergo elimination when heated with sulfuric acid via an E1
reaction to give the more stable, more highly substituted alkene as the
major product ( zaitsev's rule). A
and C are the less
stable cis isomer of B. D and E have only 5 C atoms.
Qu33: E
Need to prepare the nucleophile (an enolate) by using a suitable base,
then an SN2 reaction with the appropriate alkyl halide. D and E have sodium amide (a good base,
pKa about 35). Nomenclature ? Phenyl bromide is C6H5Br, benzyl
bromide is C6H5CH2Br. We need benzyl bromide to get the right
structure.
Qu34:B
A nucleophilic substitution of an alcohol with a carboxylate (RCO2-) as
the nucleophile... therefore need to make the -OH into a better leaving
group first.
A will give the wrong ester (it
will give propyl ethanoate or propyl acetate). C would give propene. D cause bromination of the propanol
to give a bromopropanol. There would be no useful reaction in E (no good leaving group).
Qu35: E
An ether synthesis. A
would make an ester. B and C would give no reaction. D would give the bromide in step
(1) but then no reaction (no nucleophile there) and E converts the -OH
to the tosylate, a better leaving group then substitutes it with
the methoxide as the nucleophile.
Qu36: B
Alcohol to alkene is an elimination... alcohols are eliminated
by acid...hence it's B.
Qu37: BC
IR shows a carbonyl (i.e. C=O) at 1660cm-1 which is
low for a typical ketone. The 13C-nmr peak at 176ppm supports the C=O
and suggests a carboxylic acid or derivative. This means it could
be A, B, C,
AE or BC. The H-nmr has 5 types of H, 9H
in total. No H-nmr peak near 12ppm rules out the acid AE. There is no methyl singlet in
the H-nmr so that rules out A
and B, nor is there an ethyl
group ruling out C. The H-nmr
shows a CH3CH2CH2- group and the 2 NH groups in BC - note the odd number of H atoms.
The IR C=O is consistent with the amide.
Qu38: AE
IR shows a carbonyl (i.e. C=O) at 1712cm-1 which is
typical for a ketone. The 13C-nmr peak at 181ppm supports the C=O and
suggests
a carboxylic acid or derivative. This means it could be A, B,
C, AE or BC. The H-nmr has 4 types of H, 8H
in total. The H-nmr peak near 12ppm implies the acid AE. There is no methyl singlet in
the H-nmr so that rules out A
and B, nor is there an ethyl
group ruling out C. The H-nmr
also shows the CH3CH2CH2- group.
Qu39: E
IR shows a carbonyl (i.e. C=O) at 1712cm-1 which is typical
for a ketone. The 13C-nmr peak at 181ppm supports the C=O and suggests
a carboxylic acid or derivative. This means it could be A, B,
C, AE or BC. The H-nmr has 4 types of H, 8H
in total. The H-nmr peak near 12ppm implies the acid AE. There is no methyl singlet in
the H-nmr so that rules out A
and B, nor is there an ethyl
group ruling out C. The H-nmr
also shows the CH3CH2CH2- group.
Qu40: AC
IR does not show a carbonyl (i.e. C=O) near 1700cm-1
but it does show a band at 3100-3500 which is typical for an -OH.
This means it should be AC. The H-nmr exchangeable peak at
2.6ppm supports the -OH.
Qu41: BE
IR does not show a carbonyl (i.e. C=O) near 1700cm-1 or an
-OH near 3100-3500. This limits the choices to BD and BE. Since the 13C-nmr
shows that there are 3 types of ArC (126-142ppm), then it must be BE since BD only has 2 types of aromatic
carbon.
Qu42: A
IR shows a carbonyl (i.e. C=O) at 1745cm-1 which is high for
a typical ketone. The 13C-nmr peak at 171ppm supports the C=O and
suggests a carboxylic acid or derivative. This means it could be A, B,
C, AE or BC. The H-nmr has 4 types of H, 10H
in total. No H-nmr peak near 12ppm rules out the acid AE. There is a methyl singlet in the
H-nmr at 2ppm so that rules out C,
AE and BC. The H-nmr peaks at 1.0, 1.6 and
4.1 CH3CH2CH2- group of A and
not the isopropyl group in B.
The IR C=O is consistent with the ester.