Part 7: MECHANISMS
Note that no other reagents are needed in order to complete any
of these sequences, you should only be using what is there.
Common errrors:
A: Not interpretting the Newman projection correctly. Showing an E1 pathway via a carbocation, or a substitution reaction to give an ether or an elimination with a rearrangement.
B: Using the wrong type of curly arrows. Not showing the propagation steps. Forming a primary alkyl radical that then rearranged.
C: Not showing the curly arrow for the rearrangement. Showing a concerted pathway.
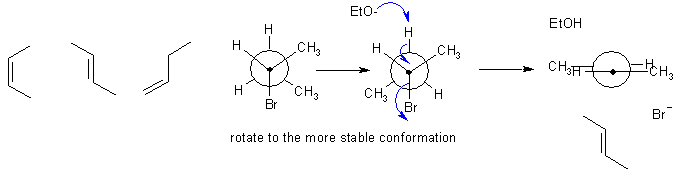
A If an alkyl bromide is heated with strong base (here it's ethoxide), then a 1,2-elimination occurs to give an alkene. Alkyl halide eliminations are typically E2. This prefers an anti arrangement of the C-H and C-LG bonds. In this reaction, the base is not really large, so the Zaistev elimination is going to dominate to give the more highly substituted alkene and the major product will be the more stable trans-alkene from the more stable conformation (methyl groups anti).

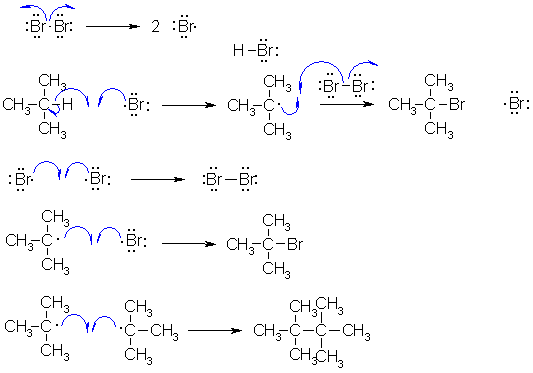
B Radical halogenation reactions. First step is the formation of the halogen radical by dissociation of the halogen. Note that the Br-Br bond is weak and readily broken when heated. The Br radical then abstracts the tertiary H to give the tertiary radical and this in turn leads to the tertiary bromide. Note that a bromine radical is also produced and this means the process can continue (it's a cyclic process). Note that because we are talking about radicals, we need to use fishhook type curly arrows.
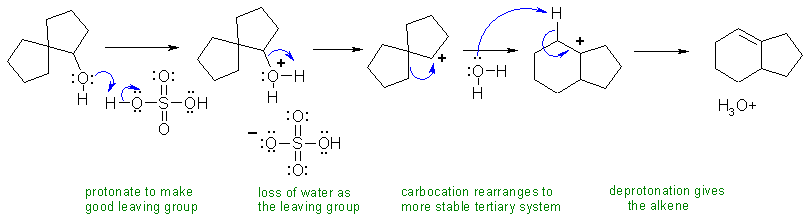
C If an alcohol is heated with strong acid, then a 1,2-elimination occurs to give an alkene. Alcohol eliminations are typically E1. In this case, we also have a rearrangement of the intermediate carbocation from a secondary to a more stable tertiary carbocation via a 1,2-alkyl shift.