
Note
that no other reagents are needed in order to complete any of these sequences,
you should only be using what is there.
A1
Bromine reacts with alkenes via an electrophilic addition mechanism, via a cyclic bromonium ion intermediate which is then opened by the attack of a nucleophilic species in this case the bromide ion. This attack of the nucleophile normally results in an overall anti addition of the two bromine atoms as is seen here with 2-butene.

Common errors: The process is not a radical reaction nor does it proceed via a carbocation. In order to avoid thes kinds of errors one needs to have a better grasp of (1) the specifics of reagents and (2) the various mechanisms. It's not like this is an uncommon or rare reaction in this course.
A2
This is a transesterification under basic conditions (based on a laboratory experiment) where the methanol deprotonates to form the reactive nucleophile methoxide that attacks the electrophilic C in the ester carbonyl group, the tetrahedral intermediate collapses to lose the other alcohol group as the leaving group. This is then protonated in the work-up. In the scheme below R and R' have been used to simplify the drawing.
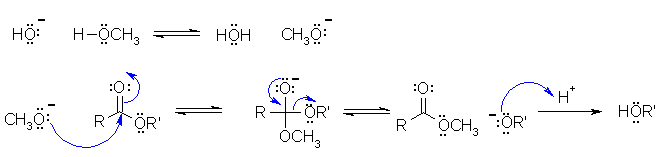
B1
This is the ring opening of an epoxide under acidic conditions (HBr). It is important to remember that in an acidic solution the epoxide will protonate before the ring is opened. This means that the epoxide opening proceeds via an SN1 like pathway where the cationic character of the epoxide C atoms in the intermediate controls where the nucleophile attacks. Therefore in this case the phenyl ring will allow for resonance stabilisation of the benzylic position and dictate the regiochemistry.
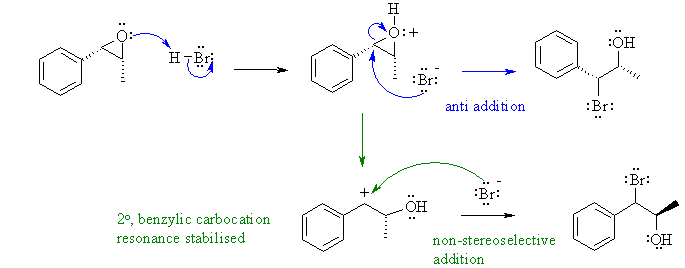
Common errors: Not protonating the epoxide before ring opening. Getting the regiochemistry wrong because the cationic character was ignored. There were a lot of statements about "attacking the more substituted end" but they are equally substituted, one none H substituent at each end. A reasonable number of students also talked about Markovnikov's rule.... but that has nothing to do with epoxides, it applies to alkenes (though it can be extended to alkynes).
B2
This is addition of HCl to an alkene involving a carbocation rearrangement (note the change in the carbon skeleton). Protonation of the C=C gives the more stable 3o carbocation is accord with Markovnikov's rule. The carbocation then rearranges via a 1,2-alkyl shift to relieve ring strain (4 membered ring to 5 membered) and the resulting 2o carbocation is attacked by the chloride ion.
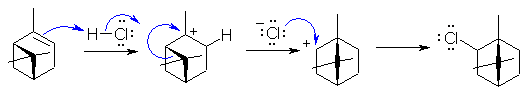
Common errors: Not protonating the alkene correctly, not showing the alkyl shift curly arrows, drawing a mechanism to a different product (??), giving a justification that was not an explanation but a restatement (i.e. saying because it's more stable is not a suitable answer, you need to say why it's more stable, calling the alkyl shift a methyl shift.
Common general errors: (1) Ignoring reaction conditions (2) poorly drawn arrows, e.g. not starting at the Nu site (3) backwards arrows (4) wrong use of arrows e.g. resonance (5) not balancing charges (6) missing arrows esp. when adding or removing H+ (7) compressing several mechanistic steps into a single step.