Note that no other reagents are needed in order to complete any of these sequences, you should only be using what is there.
Work in progress, at this time only links to similar examples are available, more detailed / specific answers will be posted as and when time allows.
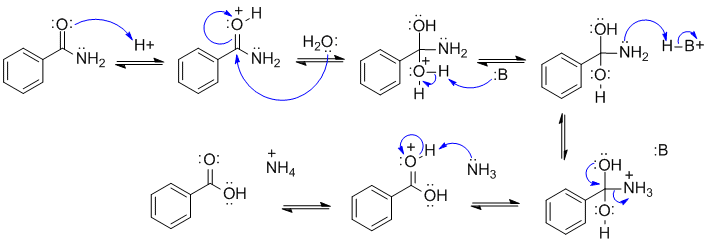
A1
This is an example of acid catalysed amide hydrolysis.

In this scheme, B: could be the chloride ion, a C=O, or N: system.
A2
This is an example of a Friedel-Crafts acylation.
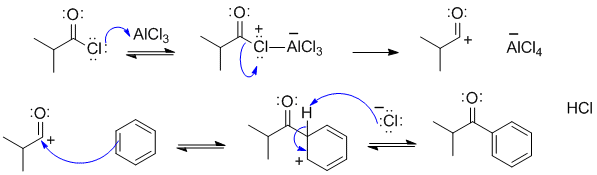
A common error in this scheme was ignoring / omitting the intermediate formed after the aromatic reacts with the electrophile, remember there is an -H that is being substituted and needs to be removed.
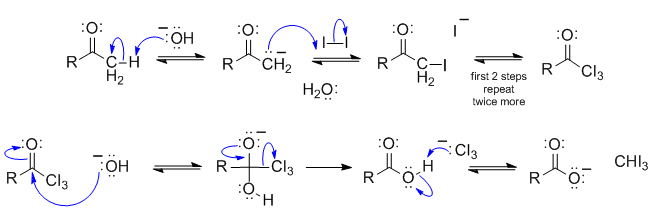
B1
This is the haloform reaction. The base reacts with the methyl ketone to form an enolate which then reacts with the iodine as a nucleophile to give the alpha iodoketone. Since the iodine makes the adjacent H more acidic, the reaction repeats (i.e. base forms enolate, enolate reacts with iodine) until you get the triiodoketone. The hydroxide now reacts as a nucleophile attacking the carbonyl giving the tetrahedral intermediate. Reform the CO double bond with loss to the leaving group (a stable anion) then the acid/base reaction give the haloform product and the carboxylate ion.
B2
The two key steps are examples of electrophilic aromatic substitutions of the benzene . In the first substitution, the electrophile is the C in the carbonyl of the ketone (activated by protonation first) and it's an example of nucleophilic addition to the ketone (weak nucleophile, so acid catalysed). In the second substitution, it's a substitution of an alcohol (acid catalyst required to make the -OH into a better leaving group: SN1). In both aromatic substitutions, the electron donating -OH group directs the reaction to the para-position.
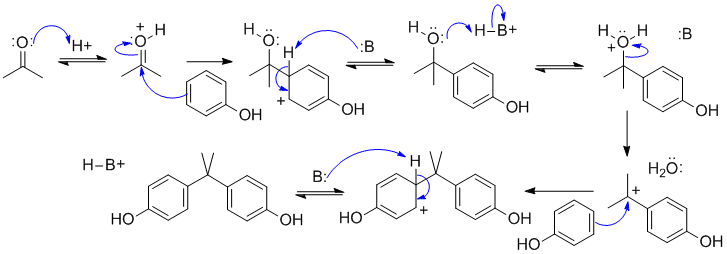
In this scheme, B: could be the conjugate base of the acid catalyst, a C=O, or O-H systems.
A common error in this scheme was ignoring / omitting the intermediates formed after the aromatics have reacted with the electrophiles, remember there is an -H that is being substituted and needs to be removed.
Common general errors: (1) Ignoring reaction conditions i.e. effect of acidic or basic environment (2) poorly drawn arrows, e.g. not starting at the Nu site (3) backwards arrows (4) wrong use of arrows e.g. resonance (5) not showing formal charges (6) missing arrows especially when adding or removing H+ (7) compressing several mechanistic steps into a single step (8) not knowing the basic mechanism types.