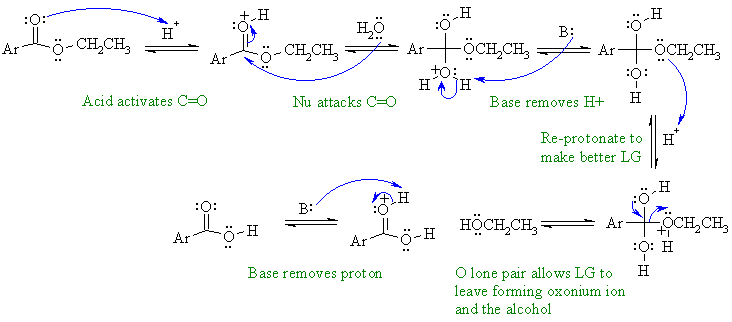
Note that no other reagents are needed in order to complete any of these sequences, you should only be using what is there.
A1
This is an example of acid catalysed ester hydrolysis.

In this scheme, B: could be water, a C=O, -OR or the conjugate base of the unspecified acid.
Common errors in this scheme not activating the carbonyl to attack by the weak nucleophile, not protonating to create the alcohole leaving group rather than the alkoxide..
A2
Nitration of an aromatic via an electrophilic aromatic substitution. First, need to show the formation of the reactive electrophile from the nitric acid / sulfuric acid mixture.
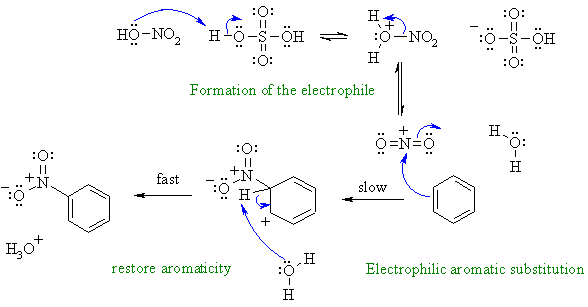
A common error in this scheme was ignoring / omitting the intermediate formed after the aromatic reacts with the electrophile, remember there is an -H that is being substituted and needs to be removed.
B1
This is the aldehyde version of the Baeyer-Villager reaction.
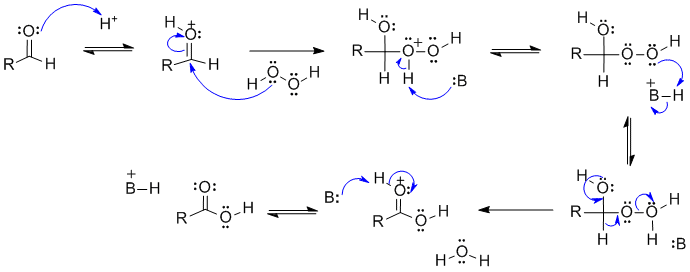
In this scheme, B: could be a C=O, -OH group or the conjugate base of the unspecified acid.
Common errors in this scheme were showing a radical reaction, not having appropriate leaving groups.
B2
LiN(iPr)2 is LDA, a strong base (pKa = 35), which will form an enolate of the ester. This enolate will then react with the ketone (similar to the aldol reaction). The product of this step contains an alkoxide and there is a good leaving group (the chlorine) on the adjacent carbon so an intramolecular Williamson type reaction - just SN2 - happens to form a cyclic ether, the epoxide (note : this last step is also like using a halohydrin to form an epoxide)
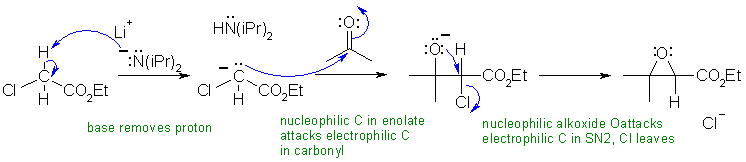
Common errors in this scheme included not recognising the strong base (It's LDA) and the source of it's electrons.
Common general errors: (1) Ignoring reaction conditions i.e. effect of acidic or basic environment (2) poorly drawn arrows, e.g. not starting at the Nu site (3) backwards arrows (4) wrong use of arrows e.g. resonance (5) not showing formal charges (6) missing arrows especially when adding or removing H+ (7) compressing several mechanistic steps into a single step (8) not knowing the basic mechanism types (9) drawing arrows that were inconsistent with the resulting bonding changes.