Note that no other reagents are needed in order to complete any of these sequences, you should only be using what is there.
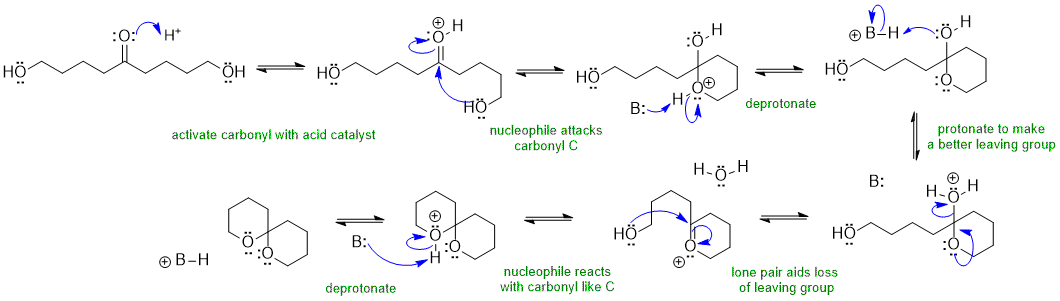
A1 Intramolecular cyclic ketal formation using a diol:

In this scheme, B: could be the conjugate base of the acid catalyst, C=O, ROH or H2O (all of which are present in the reaction solution)
Common errors:
not forming the oxonium ions (to acitvate the O atoms under acidic conditions), have HO- as the leaving group under the acidic conditions, incorrect formal charges
A2 A Dieckmann condensation:
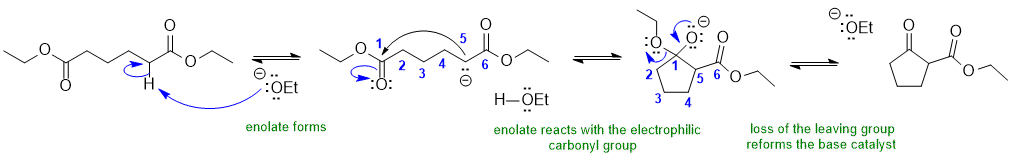
B1 Nitration, an electrophilic aromatic substitution:
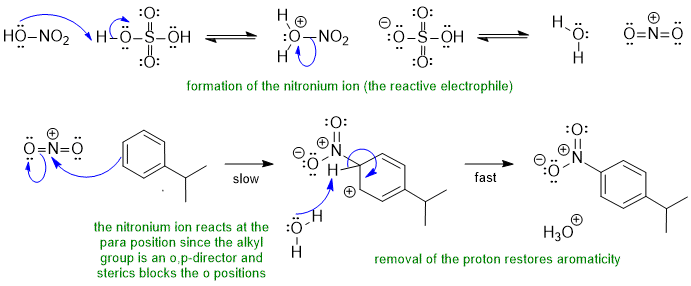
Common errors: incorrect regiochemistry, missing formal charges on intermediates
B2 Friedel-Crafts alkylation, an electrophilic aromatic substitution:
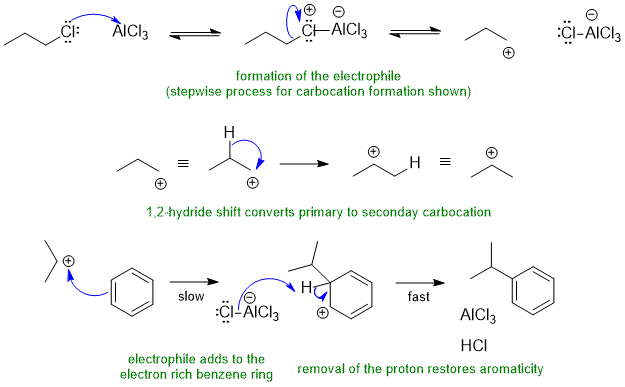
Common errors: omitting the carbocation rearrangement, not showing curly arrows for all steps.
Common general errors: (1) Ignoring reaction conditions i.e. effect of acidic or basic environment (2) poorly drawn arrows, e.g.vague (i.e. starting / ending "in space" (3) backwards arrows (4) wrong use of arrows e.g. resonance (5) not showing formal charges (6) missing arrows especially when adding or removing H+ (7) compressing several mechanistic steps into a single step (8) not knowing the basic mechanism types (9) drawing arrows that were inconsistent with the resulting bonding changes.