Chapter 6: Acidity and Basicity |
Chapter 6: Acidity and Basicity |
The acidity or basicity of a compound is controlled by a variety of structural factors that we will explore.
They can be used to help justify the relative placement of functional groups on the "rungs" of the acidity ladder.
This gives a way to rationalise the acidity and potentially predict the relative acidity of different molecules or functional groups within a molecule.
Some of the factors can be combined, there could also be multiple effects of the same type and they can overlap...
First, recall the simplified general equation of a simple acid reaction and the associated definitions:
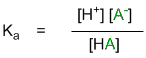 |
|||
Stabilising the conjugate base, A-, will favour dissociation of the parent acid, H-A, leading to more H+ and hence a stronger acid.
Approach : for acidity, look for factors that stabilise the conjugate base, A-
The structural factors can be used to establish the relative acidity by comparison of one system to another. We will do this using examples that relate to the groups presented on the acidity ladder.
As we do this (to begin with) it is going to be easiest if the differences between the systems are minimal. Mastering these skills will give us a chance to deal with more complex systems later.
As we explore each structural factor, we will try to give an approximate idea of how significant the impact is of this effect.
Summary of structural factors:
 |
© Dr. Ian Hunt, Department of Chemistry |