| Chapter 8: Nucleophilic Substitution |
Reaction of Alcohols with Hydrogen Halides
(review of chapter 4)
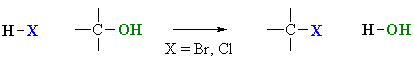
Reaction type: Nucleophilic Substitution (SN1 or SN2)
Summary:
- When treated with HBr or HCl alcohols typically undergo a nucleophilic substitution reaction to generate an alkyl halide and water.
- Alcohol relative reactivity order : 3o > 2o > 1o > methyl.
- Hydrogen halide reactivity order : HI > HBr > HCl > HF (paralleling acidity order).
- Reaction usually proceeds via an SN1 mechanism which proceeds via a carbocation intermediate, that can also undergo rearrangement.
- Methanol and primary alcohols will proceed via an SN2 mechanism since these have highly unfavourable carbocations.
- The reaction of alcohols with HCl in the presence of ZnCl2 (catalyst) forms the basis of the Lucas test for alcohols.
| SN1 MECHANISM FOR REACTION OF ALCOHOLS WITH HBr | |
|
Step 1:
Step 2:
Step 3:
|
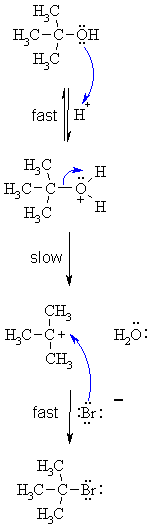 |
| © Dr. Ian Hunt, Department of Chemistry |