Part 7: MECHANISMS
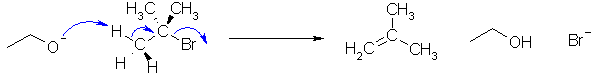
I-i The reaction that forms 2-methylpropene
from t-butyl bromide is an elimination
reaction. The reagent, sodium
ethoxide, is a strong base so we are looking at an E2
reaction : concerted reaction of the base to remove the proton,
form the C=C and loss of the bromide ion :
I-ii
The reaction that
forms
the ether,
t-butyl ethyl ether from t-butyl bromide is substitution
reaction. The
reagent, ethanol, is weak nucleophile and we have a tertiary bromide so
we are looking at an SN1
reaction so we have loss of the leaving group to give the tertiary
carbocation then attack of the alcohol as the nucleophile (not the
alkoxide - low concentration) :
I-iii Given that the
alkyl halide is quite hindered since it is tertiary, elimination is
likely the major reaction. The balance of the nucleophilicity to the
basicity of the oxygen system is also important. The alkoxide,
RONa, is very basic since the O is negatively charged and this strongly
favours elimination over
substitution. In contrast, the alcohol, ROH, is much less basic
and this disfavours elimination.
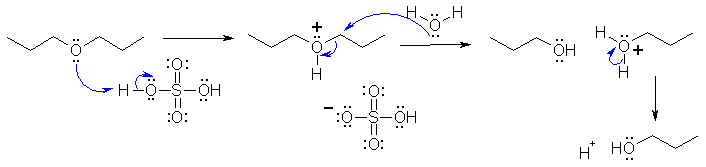
II-i The
reaction that forms 1-propanol from the di-n-propyl ether is a
substitution reaction. The reagent, 60% sulfuric acid contains water
which acts as the nucleophile. The mechanism requires that the ether O
be protonated first to make a better leaving group (-ve O groups are
poor leaving groups) and then the water attacks
to displace the leaving group in an SN2
type reaction. It will not
react as SN1 because primary
carbocations are too unfavourable.
The sulfate or bisulfate ion is a very poor nucleophile as the -ve
charge is resonance stabilised. The final step is just an acid /
base reaction to remove the proton :
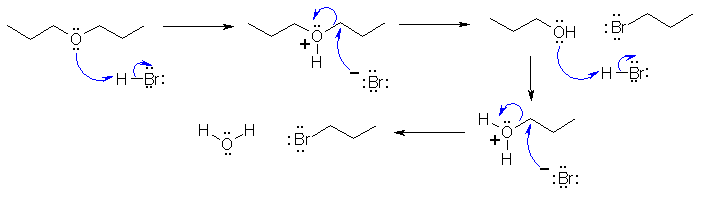
II-ii The
reaction that forms 1-bromopropane from the di-n-propyl ether is a
substitution reaction. The reagent, 60% hydrogen bromide contains the
bromide ion, a very good nucleophile. The mechanism requires that the
ether O
be protonated first to make a better leaving group and then the bromide
ion attacks
to displace the leaving group
in an SN2
type reaction. It will not
react as SN1 because primary
carbocations are too unfavourable. This forms an alcohol and an alkyl
bromide. Alcohols
react with HBr to give alkyl bromides... the mechanism requires that the alcohol O
be protonated first to make a better leaving group and then the bromide
ion attacks
to displace the leaving group in an SN2 type reaction :
III-iii In order for the
phenol to convert to bromobenzene, a substitution reaction would be
needed. But the C that would need to be attacked would be sp2
hybridised and nucleophilic substitution reactions occur on C atoms
that are sp3. Why ? SN2 - backside attack on the sp2 would
be impossible as the other C atoms in the ring are in the way. SN1 -
the phenyl cation is too unstable to form.