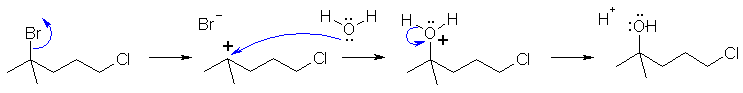
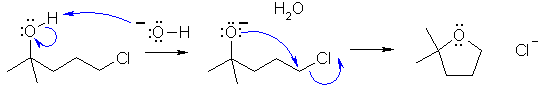
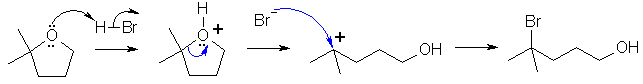
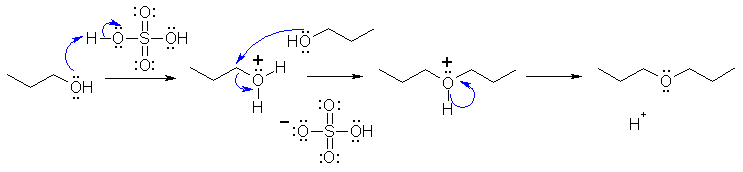
Cyclohexanol is reactive to both
nucleophile substitution and elimination while phenol is not.
Consider each of the possible mechanisms...
SN1 and E1: These reactions have a common rate determining step,
carbocation formation. The phenol would require the formation of
a very unfavourable phenyl cation. Hence phenol doesn't undergo Sn1 or
E1 reactions.
SN2 : This requires backside attack. The planar nature of the phenol
prevents this. Cyclohexanol is not planar, the OH is on a
tetrahedral, sp3 C, so the nucleophile can attack.
E2 : These eliminations require that the H and the LG be coplanar, most
commonly anti (180 degrees) but syn (0 degrees) is an alternative. The
phenol could react via a syn elimination to give benzyne (see above) -
but this is a very reactive compound (but it is known).