Part 7: MECHANISMS
Note that no other reagents are needed in order to complete any of these
sequences, you should only be using what is there.
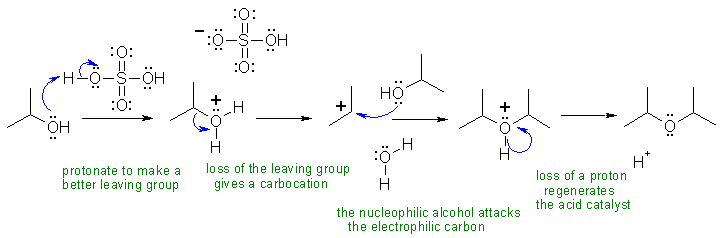
I Alcohols can be both the electrophilic side and the nucleophilic side of substitution reactions. In this example, they are both ... this can be used for the synthesis of symmetrical ethers.

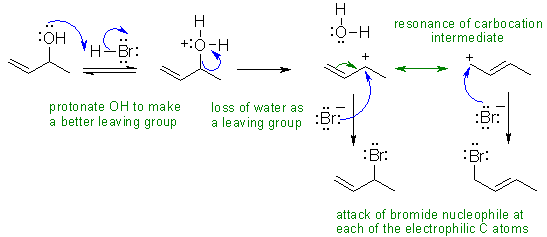
III If an alkyl bromide is heated with strong base (here it's hydroxide), then a 1,2-elimination to give an alkene occurs. Alkyl halide eliminations are typically E2. In this case the base is small so the Zaistev elimination is going to dominate. E2 reactions prefer an anti arrangement of the H and the leaving group - this dictates the stereochemistry of the alkene product.

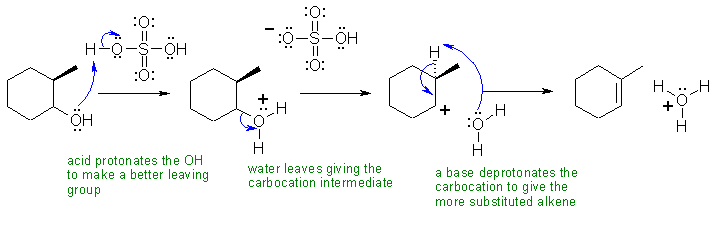
IV Both reactions are eliminations, but the alcohol dehydration is E1 and the alkyl halide dehydrohalogenation is E2. E1 reactions tend to give the more stable (more highly substituted alkene as the major product = Zaitsev's rule) while E2 are controlled other factors including the preference for an anti arrangement of the H and the leaving group.
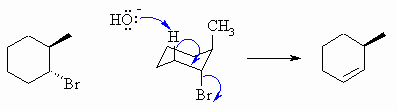
The base removes the H at 180o to
the Br leaving group, this defines the alkene location.