Chem 351 Final Fall 2014
Here is an post-mortem analysis / "how to" for
the FINAL. The questions are split by the sections. At the start of each section
are a few suggestions of what to look for or how to tackle the question type.
RELATIVE PROPERTIES:
Identify the controlling feature,
which is not always as obvious as
it may appear. Look for two pairs of similar systems to compare that
have
minimal differences in structure.
| Qu1: |
A question about stability carbocations. Remember that phenyl carbocations are quite unstable while resonance stabilises carbocations (e.g. benzylic systems (ii) are resonance stabilised). |
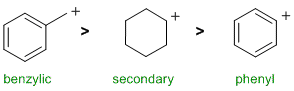 |
| Qu 2: |
All about the nucleophilicity of these molecules. All the systems are neutral, but it is the lone pairs on the O and N that make those molecules nucleophilic. There is an oxygen systems and a nitrogen system, all are single
bonded. Let's deal with the O and N first. While there are two lone pairs on the O versus one on the N, the lower electronegativity of the N makes it a better electron donor (more nucleophilic). There are no available electrons on the alkane, it's a very poor nucleophile. |
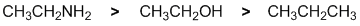 |
| Qu 3: |
Leaving group ability....remember that good leaving groups need to be stable when they leave and therefore tend to be the conjugate bases of strong acids. Here we have a bromide, an alcohol and an iodide. Since HI is stronger than HBr, that tells us the I- is more stable than Br- and so I- is a better leaving group. Since H2O is a weak acid, HO- is a very poor leaving group. |
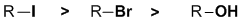 |
Qu 4: |
Basicity...either think about the availability of the electrons in the base or the stability
of the bases (look for the lone pairs) or relate to the pKa's of the appropriate systems. The stronger the bases, then the more the reaction shown moves to the right. These are all based on atoms from the first row of the periodic table, but one is -ve (more electron rich, more basic). There are no available electrons on the alkane, it's a very poor base. |
 |
Qu 5: |
First identify the reaction.... the conditions of NaI/ acetone suggest an SN2 reaction of alkyl halides (think back to the laboratory expt). A quick look at the systems shows two bromides and an alcohol. HO- is a poor leaving group, so it will not react with NaI (hence the least reactive). Since the reaction is SN2, primary systems tend to be more reactive than secondary (due to steric hinderance). |
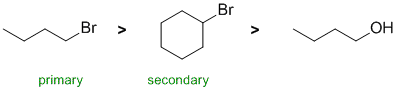 |
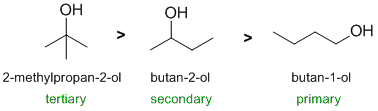
Qu 6: |
First identify the reaction.... the alcohol starting materials and reaction conditions of HBr suggest formation of an alkyl bromide via an SN1 reaction. These reactions are typically controlled by the stability of the intermediate carbocations. Probably a good idea to draw out the structures and look at the nature of the carbocation being formed. More stable carbocations form faster thnd that dicates the reaction rate 3o > 2o > 1o |
 |
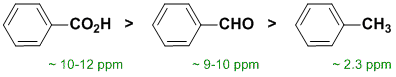
Qu 7: |
Chemical shifts of the groups in question in these systems are determined by the nature of the groups attached to the methyl group (that is what is changing). Chemical shifts are affected by electronegative atoms (especially such as O) and magnetic anisotropy (due to pi systems). |
 |
| Qu 8: |
Acidity.... Need to look at the stability of the conjugate bases and consider the factors that stablise that. H attached to more electronegative atoms tend to be more acidic (the electronegative atom stabilises the conjugate base) so since O more electronegative than C, O-H are more acidic than similar C-H atom (as in CH3Br) in the periodic table. H on larger atoms tend to be more acidic, S is in the second row below O (S more able to accept -ve), so S-H is more acidic that O-H. A knowledge of the pKa values of common acids really helps! |
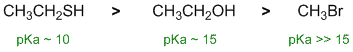 |
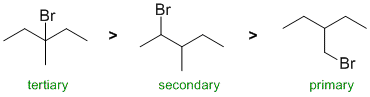
| Qu 9: |
This is a radical substitution reaction. With bromination, the reaction is much more selective for the more stable radicals (i.e. tertiary > secondary > primary). |
 |
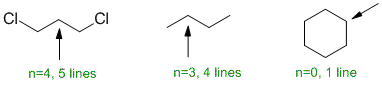
| Qu 10: |
The number of lines = multiplicity (i.e. coupling) of the signals for each of the positions indicated is determined by the number of neighbours (n) that are of a different type (since H of the same type do not show coupling e.g. the CH2 groups in cyclohexane (where there is only 1 type of H). |
 |
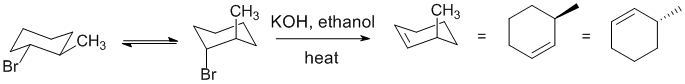
| Qu 11: |
The question is about elimination reactions. Alkyl halides eliminate with strong base / heat. The Zaitsev product is favoured most with smaller (less bulky) bases. |
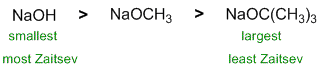 |
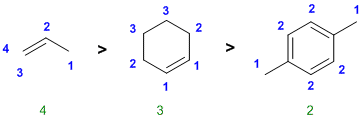
| Qu 12: |
Counting types of hydrogen |
 |
MOLECULAR PROPERTIES
No real method here, really just do you
know various aspects of molecular structure / reactions, applied to each of the questions.
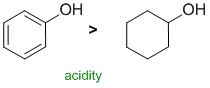
| Qu 13: |
Alcohols react with NaOH in acid / base reactions. The most acidic will be the most reactive, the most acidic will be the system with the most stable conjugate base. The phenol II has a resonance stabilised conjugate base and is therefore more acidic (pKa = 10) compared to cyclohexanol (pKa ~ 15). |
 |
| Qu14: |
The Cahn-Ingold-Prelog priority rules are based on atomic number at the first point of difference. Let's look at what the attached atom is and what atoms that is attached to (listed by atomic number). So I is a C (C,H,H) and II is a C (C,C,C) which means that both attach via a C atom, but when we look an atom further away, we see a difference where the C outranks the H ( I = C (C,H,H) and II = C (C,C,C)). |
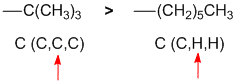 |
| Qu15: |
As their name implies, amino acids contain amines and carboxylic acids. Amines are basic and carboxylic acids are acidic enough to protonate them. So, in aqueous solution, an amino acid will exist in its zwitterionic form II |
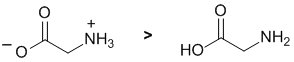 |
| Qu16: |
The formation of a tosylate from an alcohol does not change the C-O bond (i.e. the C-O in the tosylate is the same C-O bond as in the alcohol). A secondary tosylate with reaction with NaBr in an SN2 reaction which cause an inversion of stereochemistry. |
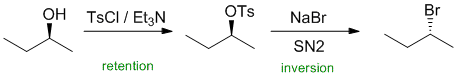 |
| Qu17: |
Easiest to answer by pushing the curly arrows to derive the resonance contributor. The most important factor in ranking resoance structures is the octet rule. |
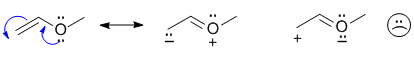 |
| Qu18: |
Easiest to answer by pushing the curly arrows from X to derive the resonance contributor. |
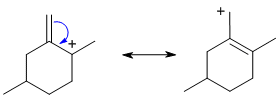 |
REACTIONS:
Three types of questions.
For those
with starting materials work from
the starting materials towards the products using the reagents to "see"
what product to look for.
For those with the product work
backwards....
looking at the functional groups in the products to think about how you
may have got there.
For those wanting reagents look at
the functional groups in the
starting material and products
to try to determine what may have happened. Look at the reagents in
each
option to see what effect they would have on the SM....
| Qu19: |
We should work forwards...looking at the starting material and the reagents, we need to convert the alkane to an alkyl chloride via a radical substitution, followed by an E2
elimination of the alkyl halide with a bulky base to give the anti-Zaitsev product (less highly substituted). The methylcyclobutane will radically chlorinate to give 1-chloro-2-methylcyclobutane (4 secondary H outranks 1 tertiary H i.e. (4 x 3.9) > (1 x 5.2)), which then eliminates with the bulky base to give 3-methylcyclobutene. |
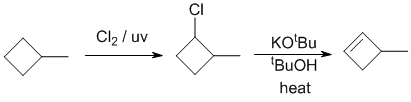 |
| Qu20: |
We should work forwards...starting from an alcohol and going to an ether, but notice that -O- is in a different location (telling us there has been a rearrangement) meaning SN1 reactions are involved. Here, the alcohol is made into a better leaving group with an acid (promoting SN1 via C+) and methanol reacts as a nucleohile with the C+ to give the methoxy ether. |
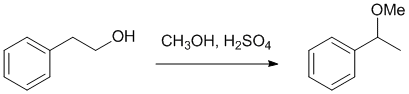 |
| Qu21: |
We should work forwards.... starting from an alcohol and going to an alkene would normally use something like H2SO4 / heat.... not there.... the alternative would be to make the alcohol into an alkyl halide via halogenation of an alcohol using thionyl chloride and then use a base to eliminate the alkyl halide. Since the alkene is the Zaitsev (more substituted) alkene, a base like HO- is a good choice. |
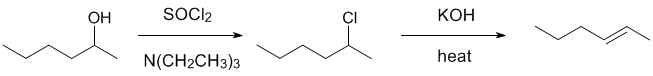 |
| Qu22: |
We should work forwards ....Alcohols react with acid catalysts to convert the -OH in to a better leaving group. If a Nu is present, nucleophilic substitution can occur, especially at lower temperatures. In this case, the Nu is just the alcohol itself, so the product is a symmetrical ether. |
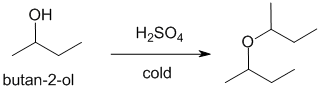 |
| Qu23: |
Easiest to answer by pushing the curly arrows to derive the resonance contributor. The most important factor in ranking resoance structures is the octet rule. |

|
| Qu24: |
Easiest to answer by pushing the curly arrows from X to derive the resonance contributor. |
 |
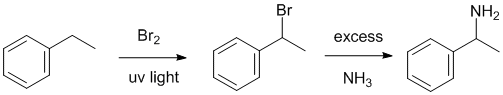
| Qu25: |
We need to work backwards.... The amine has been made using excess ammonia via a nucleophilic substitution likely from a bromide (given step 1). The bromide has been made via radical bromination will give the benzylic bromide as the major product. Count C atoms, we need the ethyl group. |
 |
| Qu26: |
We should work forwards ....starting from the ketone and looking at the product we need to add 2C atoms in an alkyl group. We can do this via nucleophilic substitution if we make the ketone in to a better nucleophile (by deprotonating using a base) and then reacting it with Et-LG (e.g. Et-Br). |
 |
CONFORMATIONAL ANALYSIS:
Understanding the terminology and the
energies involved in
conformational
analysis.
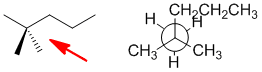
Qu 27:
Draw out the 2,2-dimethylpentane (C7H16).... this reveals the presence of a t-butyl unit which you can look for. On the other hand, only C is C7.
Qu 28:
The torsional angle between the fluorine atoms is 120 degrees in this eclipsed conformation.
Qu 29:
All the structures drawn are all 1,2-dimethylcyclohexane. Cis requires that the two methyl groups are on the same face of the ring.

Qu30:
While the Newman projection shows a staggered conformation, it shows the two indicated bonds at 60 degrees and hence gauche (this is the best, i.e. most complete term)
Qu31:
The structures are isomeric (same MF) but what type of isomer are they? Comparing the drawings or picking up one model and comparing allows one to they are identical.
Qu32:
All the structures show 1,3-dimethylcyclohexane. The most stable conformation will be i. a chair (A, B, C) and ii. have both methyl groups equatorial.
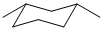
Qu33:
The chair conformation of cyclohexane D is staggered and hence has minimal torsional strain (it's regarded as being strain free).
Qu34:
Need to assign the configurations based on the Cahn-Ingold-Prelog rules for the alkene stereochemistry and the chirality center.
For the alkene (rankings shown in blue), consider the two ends separately. Towards the acid group, the C in the CH2 attached out ranks the H. At the other end, the C (C,C,H) outranks the C (C,H,H). So the two higher priority groups are on the same side, and hence this is a Z isomer (German; zusammen = together).
For the chirality center (rankings shown in red), the groups in priority order are OH > C(O,O,O) > C(C,H,H) > H. Remember to add the C-H as a wedge (the lowest priority group) which is towards us, and account for this when assigning the configuration.
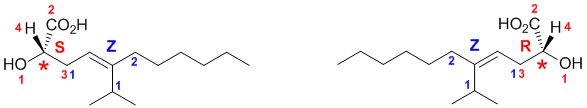
SPECTROSCOPY:
Use any IR information to get the
functional groups. Use the H-NMR
to get the number of types of H, how many of each type from the
integral
and what they are next to from the coupling patterns. Chemical shifts
should
tell you if the group is near -O- or maybe C=O groups etc.
![[Home]](../mol.gif) Return to homepage
Return to homepage




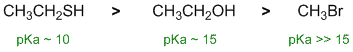


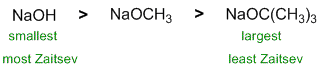


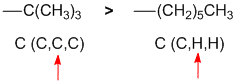
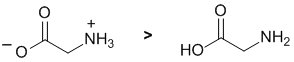




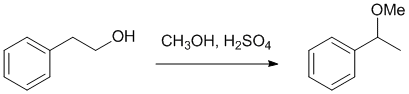









![[Home]](../mol.gif)