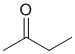
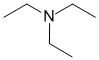
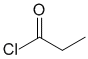
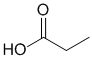
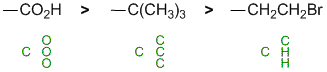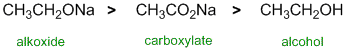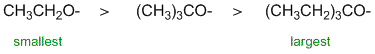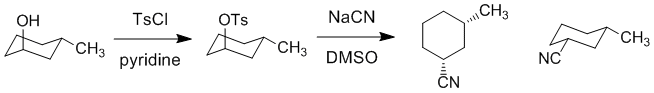Chem 351 Final Fall 2015
Here is an post-mortem analysis / "how to" for
the FINAL. The questions are split by the sections. At the start of each section
are a few suggestions of what to look for or how to tackle the question type.
RELATIVE PROPERTIES:
Identify the controlling feature,
which is not always as obvious as
it may appear. Look for two pairs of similar systems to compare that
have
minimal differences in structure.
| Qu1: |
A question about stability carbocations. Remember that the order of stability is tertiary > secondary > primary and that allylic carbocations are further stabilised by resonance. |
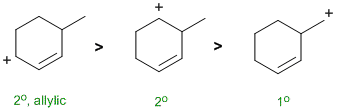 |
| Qu 2: |
The Cahn-Ingold-Prelog priority rules are based on atomic number at the first point of difference. All are based on C attachments, but O > C > H to assign the priorities. |
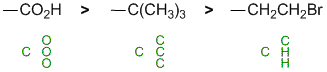 |
| Qu 3: |
Really a question about alkane stability. Notice they are isomeric (C8H18). The more stable the alkane the less exothermic (less negative) it's heat of combustion. In terms of alkane stability, the general rule is that a more branched structure tends to be more stable (note that they have more primary CH bonds which are stronger and hence more stabilising). |
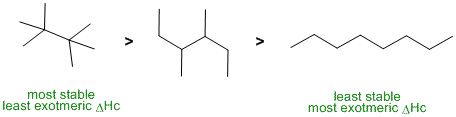 |
Qu 4: |
Acidity.... Need to look at the stability of the conjugate bases and consider the factors that affect that stabilisation. Remember that the lower the pKa the stronger the acid). Here we have substituted carboxylic acids (typical general pKa about 5) and a simple thiol (pKa = 10). The acids are more acidic than the thiol because of the resonance delocalisation of the carboxylate ion. Now we need to separate the two substituted acids. The difference in the structure is the adjacent halogen. The more electronegative that adjacent atom, the more it helps spread out the -ve charge through inductive effects. A knowledge of the pKa values of common acids really helps!
|
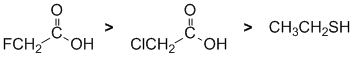 |
Qu 5: |
Acidity.... Glycine is the simplest amino acid. In a solution of pH = 2 (quite acidic), the major species will have all the sites will be protonated, but a minor species where the more acidic carboxylic acid (glycine pKa = 2.34) has deprotonated will also exist. |
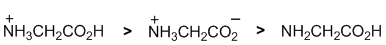 |
Qu 6: |
First identify the reaction.... the alcohol starting materials and reaction conditions of H2SO4 suggest alcohol dehydration via an E1 reaction. These reactions are typically controlled by the stability of the intermediate carbocations. Probably a good idea to draw out the structures and look at the nature of the carbocation being formed. More stable carbocations form faster and that dicates the reaction rate 3o > 2o > 1o |
 |
Qu 7: |
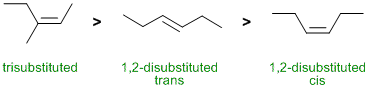
Alkene stability. Notice they are isomeric, C6H10. In terms of alkene stability, the general rule is the more alkyl groups on the C=C unit, the more stable it is and for chain systems, trans tend to be more stable than cis. |
 |
| Qu 8: |
All about the nucleophilicity of these molecules. All of the systems based on O atoms, two are negative O and the other is neutral (the Na indicates we have ionic compounds and hence the -ve charges on the O). When we are dealing with the same central atom, -ve systems are more nucleophilic than neutral systems (greater e availability). Of the two -ve systems, one has resonance delocalisation which stabilises the charge and reduces the nucleophilicity hence the alkoxide is more nucleophilic than the carboxylate. |
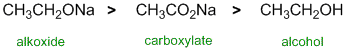 |
| Qu 9: |
This is a radical substitution reaction of alkanes (i.e. sp3 C-H bonds), remember the laboratory experiment ? For chlorination, the reaction is controlled by a combination of the relative reactivity of the radical formed and the number of H that yield that radical. First note that sp2 CH bonds don't undergo this reaction (bonds are too strong). Then while simple tertiary systems are 5.2 times more reactive than primary systems, but here is have a more stable benzylic radical. |
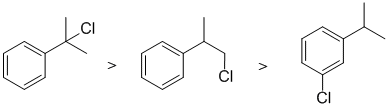 |
| Qu 10: |
Chemical shifts of the groups in question in these systems are determined by the nature of the groups attached to the methyl group (that is what is changing). Chemical shifts are affected by electronegative atoms (especially such as O) and magnetic anisotropy (due to pi systems). |
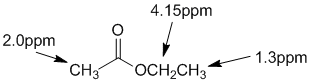 |
| Qu 11: |
The question is about elimination reactions. Alkyl halides eliminate with strong base / heat. The Zaitsev product (more highly substituted product and more stable alkene) is favoured most with smaller (less bulky) bases. |
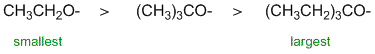 |
| Qu 12: |
Carbonyl IR stretching frequencies based on functional groups... ketones are the "normal" value of 1715 cm-1. The resonance electron donation of the N in the amide lowers the frequency while the electronegative effect of the Cl raises the frequency. |
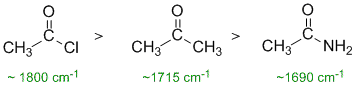 |
MOLECULAR PROPERTIES
No real method here, really just do you
know various aspects of molecular structure / reactions, applied to each of the questions.
REACTIONS:
Three types of questions.
For those
with starting materials work from
the starting materials towards the products using the reagents to "see"
what product to look for.
For those with the product work
backwards....
looking at the functional groups in the products to think about how you
may have got there.
For those wanting reagents look at
the functional groups in the
starting material and products
to try to determine what may have happened. Look at the reagents in
each
option to see what effect they would have on the SM....
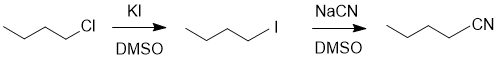
| Qu19: |
We should work forwards...KBr suggests a nucleophilic substitution to give a bromide which then reacts with ammonia to give the amine. Count C atoms and look for a leaving group. |
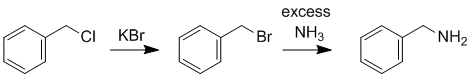 |
| Qu20: |
We should work backwards... The nitrile has been made via a a nucleophilic substitution of a tosylate which means we started with an alcohol. Since the substitution of the tosylate is an SN2 reaction there will be an inversion of stereochemistry at that center. Since the product is cis, the starting alcohol needs to be trans.
|
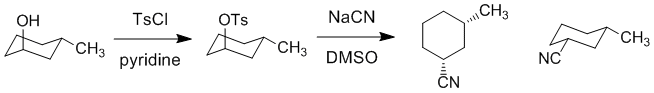 |
| Qu21: |
We should work forwards.... starting from an alcohol and going to an alkene (E1 dehydration) would normally use something like H2SO4 / heat.... Since the alkene is the Zaitsev (more substituted) alkene, H2SO4 / heat is a good choice. |
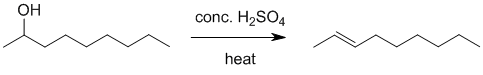 |
| Qu22: |
We should work forwards ....An alkyl halide reacts with strong base / heat to undergo an E2
elimination of the alkyl halide. For cyclohexane systems, this means that the leaving group and adjacent H need to be axial which can dicate the regiochemistry. |
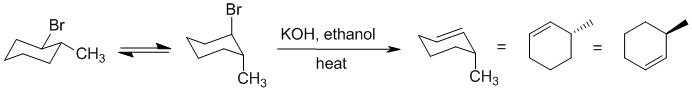 |
| Qu23: |
We should work forwards....Alcohol to alkyl halide where the location of the functional group has changed suggests a carbocation rearrangement has occurred and therefore we require SN1 reactions, i.e. react with an acid (HBr). Here, the alcohol is made into a better leaving group with an acid (promoting SN1 via C+ with hydride shift) and the bromide ion reacts as a nucleohile with the C+ to give the alkyl bromide. |
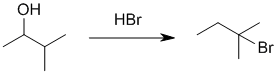
|
| Qu24: |
We should work forwards.... An E2
elimination of the alkyl halide with a base to give the Zaitsev product (more highly substituted). Need to rotate the system to put the H and the Br at 180 degrees to deduce the stereochemistry of the product. |
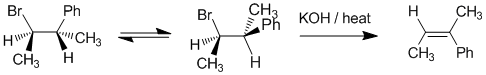 |
| Qu25: |
We need to work backwards.... The bromide has been made via radical bromination will give the allylic bromide as the major product. The first step suggests alcohol giving the alkene (E1 dehydration). |
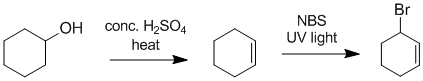 |
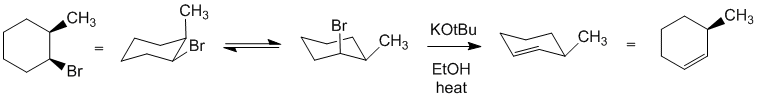
| Qu26: |
We should work forwards.....Alcohol to alkyl halide where the location of the functional group has remained unchanged despite being next to a branch point means we need to avoid C+ and therefore we require an SN2 reaction, i.e. avoid acids. Here, the alcohol is made into a better leaving group and substituted with thionyl chloride to give the alkyl chloride. |
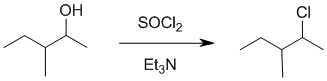 |
CONFORMATIONAL ANALYSIS:
Understanding the terminology and the
energies involved in
conformational
analysis.
Qu 27:
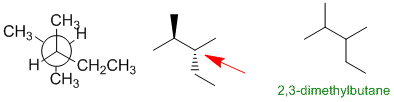
Draw out the 2,3-dimethylpentane (C7H16).... this reveals the presence of an isopropyl unit which you can look for. Also note that one of the systems is C6.
Qu 28:
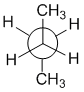
The torsional angle between the fluorine atoms is 60 degrees in this staggered and gauche conformation.

Qu 29:
Only 3 of the 5 structures drawn are all 1,3-dimethylcyclohexane (two are 1,4-). Trans requires that the two methyl groups are on opposite faces of the ring.
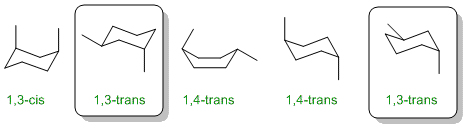
Qu30:
The chair conformation of a subsituted cyclohexane is a staggered conformation. As a result, the two methyl groups are at 60 degrees and hence gauche (this is the best, i.e. most complete term)
Qu31:
The structures are isomeric (same MF) but what type of isomer are they? Comparing the drawings or using models and comparing allows one to determine that they are non-superimposable mirror images and hence enantiomers.
Qu32:
All the structures show 1-t-butyl-3-methylcyclohexane. The most stable conformation will be a chair and will have either both alkyl groups equatorial or, if not, the larger t-butyl group equatorial.
Qu33:
All are butane... look for the staggered conformation with the methyl groups anti (180o).
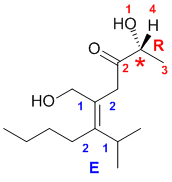
Qu34:
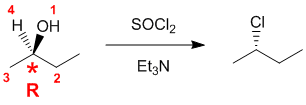
Need to assign the configurations based on the Cahn-Ingold-Prelog rules for the alkene stereochemistry and the chirality center.
For the alkene (rankings shown in blue), consider the two ends separately. Towards the alcohol group, the C in the CH2 attached out ranks the H. At the other end, the C (C,C,H) outranks the C (C,H,H). So the two higher priority groups are on the same side, and hence this is a E isomer (German; entgegen = opposite).
For the chirality center (rankings shown in red), the groups in priority order are OH > C(O,O,C) > C(H,H,H) > H. Remember to add the C-H as a wedge (the lowest priority group) which is towards us, and account for this when assigning the configuration (R).
SPECTROSCOPY:
Use any IR information to get the
functional groups. Use the H-NMR
to get the number of types of H, how many of each type from the
integral
and what they are next to from the coupling patterns. Chemical shifts
should
tell you if the group is near -O- or maybe C=O groups etc.