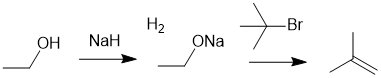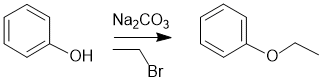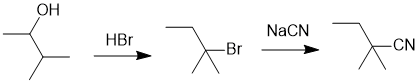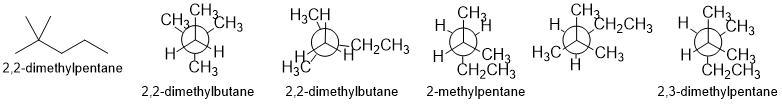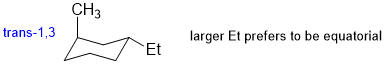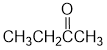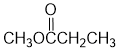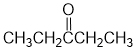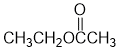Chem 351 Final Fall 2019
Here is an post-mortem analysis / "how to" for
the FINAL. The questions are split by the sections. At the start of each section
are a few suggestions of what to look for or how to tackle the question type.
RELATIVE PROPERTIES:
Identify the controlling feature,
which is not always as obvious as
it may appear. Look for two pairs of similar systems to compare that
have
minimal differences in structure.
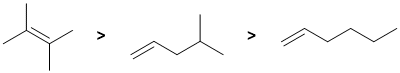
| Qu 1: |
Alkene stability is controlled by the degree of substitution of the C=C where more substituted is more stable. Therefore a tetra-substituted alkene is more stable than a mono-substituted alkene. In terms of the two mono-substituted alkenes, it is the branched system that is more stable. |
 |
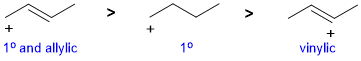
| Qu 2: |
A question about stability carbocations. Remember that the order of stability is tertiary > secondary > primary > methyl and that allylic carbocations are further stabilised by resonance...but vinyl cations are less stable than primary. |
 |
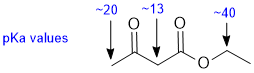
| Qu 3: |
Acidity.... Need to look at the stability of the conjugate bases and consider the factors that affect that stability. All are CH systems and it is the proximity to adjacent C=O where a resonance interaction will further stabilise the conjugate bases that makes a post ion more acidic and two adjacent C=O is better than one. The pKa adjacent to a ketone is about 20, a 1,3-ketoester is about 13 while next to an alkoxy group is about 40 (remember a lower pKa implies a stronger acid). |
 |
Qu 4: |
First identify the reaction... SN2.... which will tend to be controlled by the degree of substitution where the general trend is that primary > secondary > tertiary. Of the two secondary systems, the better leaving group (iodide) will be more reactive. |
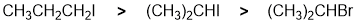 |
Qu 5: |
Basicity.... the strongest base will produce the most the greatest amount of the conjugate base. The strongest base is the amide ion (associated pKa = 35) then alkoxide (pKa approx. 15) and lastly the tertiary amine (pKa about 10).... for basicity, think about where the e are coming from ? -ve more available than neutral and the electronegativity of the atom (same row) carrying the lone pairs will affect electron availability. |
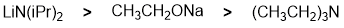 |
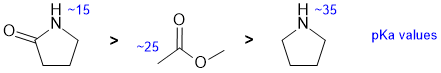
Qu 6: |
Acidity.... Need to look at the stability of the conjugate bases and consider the factors that affect that stability. Here we have two N-H and one CH system. There is also the effect of the proximity to adjacent C=O where a resonance interaction will further stabilise the conjugate bases that makes a position more acidic. The pKa of N-H in an amide is about 15, adjacent to an ester is about 25, and an amine NH is about 35 (remember a lower pKa implies a stronger acid). |
 |
Qu 7: |
First identify the reaction...alcohol dehydration.... which will tend to be E1 and therefore the stability of the intermediate carbocation. Recall that for C+ stability tertiary > secondary > primary (due to e donating properties of alkyl groups). |
t-butanol > sec-butanol > n-butanol |
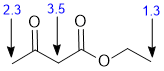
| Qu 8: |
Chemical shifts of the groups in question in these systems are determined by the position of the H (that is what is changing). Chemical shifts are affected by electronegative atoms (especially such as O) and magnetic anisotropy (due to pi systems). The methyl CH3-C=O is slightly deshielded by the proximity of the carbonyl group (about 2.3 ppm), the methylene between the two C=O is more deshielded (about 3.5ppm). The methyl group in the ethyl group is the least deshielded (about 1.3ppm) |
 |
| Qu 9: |
The IR stretching frequency of a C=O is governed by the nature of the attached groups in the specific functional group. A ketone is the base / typical value at 1715 cm-1. Esters are a little higher (about 1735 cm-1) due to the increased double bond character due to the electronegativity of the O, while the anhydride in much higher (1810 & 1760) due to the electron withdrawing character of a carbonyl group. |
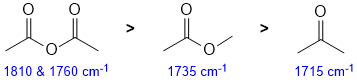
|
| Qu 10: |
Counting types of carbon |
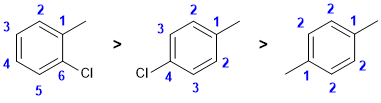 |
| Qu 11: |
The number of lines = multiplicity (i.e. coupling) of the signals for each of the positions indicated is determined by the number of neighbours (n) that are of a different type and the n+1 rule. Here we see 6 lines (5 neighbours), a triplet (2 neighbours) and a quartet (3 neighbours). |
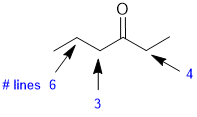 |
| Qu 12: |
First identify the reaction...dehydrohalogenation of alkyl halides using strong base and heat which will tend to be E2. The Zaitsev product is favoured by smaller bases due to the accessibility of the H that needs to be removed so bulky bases will favour the anti-Zaitsev product. |
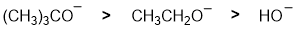 |
MOLECULAR PROPERTIES
No real method here, really just do you
know various aspects of molecular structure / reactions, applied to each of the questions.
| Qu13: |
The reaction is an SN process. HBr and allyl alcohol will be an SN1 (C+ is stable). PBr3 is typically SN2. NaBr will not react with an alcohol due to the lack of a good leaving group. |
| Qu14: |
The Cahn-Ingold-Prelog rules are based on the higher atomic number at the first point of difference: i.e. C (C,C,C) > C (C, H, H). |
| Qu15: |
A tertiary halide is more likely to substitute at 25C via an SN1 pathway especially with EtOH which is only a weak base. |
| Qu16: |
The C in the C=O of the carboxylic acid is deshielded more when the Br atom is closer (due to the electronegativity of the Br). |
| Qu17: |
Elimination of alcohols with acid is typically E1 and prone to rearrangement and the formation of the Zaitsev product while alkyl bromides typically undergo E2. |
| Qu18: |
Push the curly arrows! Two of the choices are valid resonance contributors (check overall charge and changes to sigma bonds). |
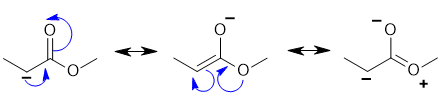 |
REACTIONS:
Three types of questions.
For those
with starting materials work from
the starting materials towards the products using the reagents to "see"
what product to look for.
For those with the product work
backwards....
looking at the functional groups in the products to think about how you
may have got there.
For those wanting reagents look at
the functional groups in the
starting material and products
to try to determine what may have happened. Look at the reagents in
each
option to see what effect they would have on the SM....
| Qu19: |
We should work forwards.... Sodium hydride reacts as a base with the acidic H in the alcohol to create the alkoxide ion and hydrogen gas. The alkoxide will then react as a base when reacted with t-butylbromide (sterically hindered) via an E2
elimination reaction to give an alkene. |
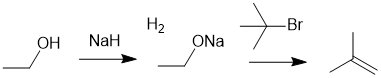 |
| Qu20: |
We should work forwards and review the transformation by comparing starting material and product. The alcohol is being converted into an ethyl ether. This would be achieved via an SN2 reaction of an O nucleophile for which we need a base and ethyl bromide. Remember that the starting materials for SN reactions have sp3 C. |
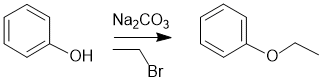 |
| Qu21: |
We should work forwards... the reaction is an SN2 involving the creaction of an ester enolate (a nucleophile) using a strong base (e.g. LiN(iPr)2 to deprotonate adjacent to the ester then adding the allyl bromide (need a good leaving group). |
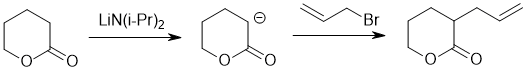 |
| Qu22: |
We should work forwards ...an alcohol to the cyanide at a more substituted position means we need to use a nucleophilic substitution via SN1 (to get the required carbocation rearrangement).
Therefore, convert the -OH into the rearranged tertiary -Br using HBr and then substitute the -Br for the -CN using a cyanide ion. |
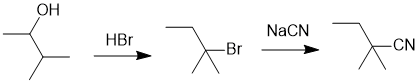 |
| Qu23: |
We should work forwards... The required alkene is the less highly substituted anti-Zaitsev alkene and therefore E2 will be required so we need to convert the alcohol to a halide and then use a bulky base. |
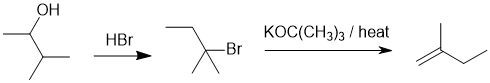
|
| Qu24: |
We should work forwards...the alkane is being radically chlorinated to give an alkyl chloride and then converted to a methyl ether via an SN1 (with a rearrangement). |
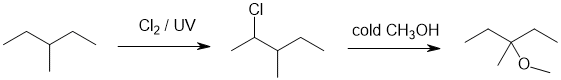 |
| Qu25: |
We can work backwards...the amine has been made from a chloride which in turn came from an alcohol. Conversion of the alcohol to the alkyl halide using thionyl chloride via a nucleophilic substitution and then a nucleophilic substitution with an amine as the nucleophile to give a amine. Both reactions are SN2, so there is an inversion followed by an inversion so we have overall retention of stereochemistry and this defines the stereochemistry of the starting material. |
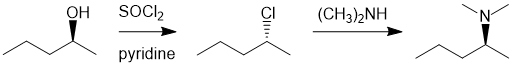 |
| Qu26: |
We should work backwards.... the product is an alkene formed using strong base and heat (an E2
elimination) of a tosylate. Given the cyclic nature, and anti-Zaitsev alkene (less substituted) product, you need to consider the stereochemical preference for the H-C-C-Cl to be at 180 degrees which requires that the Cl and H be in axial positions. We also need to ensure that the starting material gives only the enantiomer shown. |
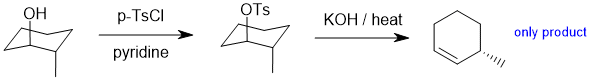 |
CONFORMATIONAL ANALYSIS:
Understanding the terminology and the
energies involved in
conformational
analysis.
Qu 27:
First, draw the named structure, then to help, count C atoms and check the arrangement of the methyl groups (t-butyl group is a good thing to look for) when you match it to the Newman projection.
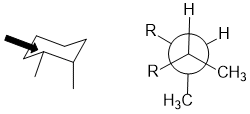
Qu 28:
The torsional angle between the two methyl groups is 60 degrees in this chair conformation of cis-1,2-dimethylcyclohexane.
Qu 29:
Make sure that the Cl atoms are 1,4- and are trans which requires that the two chlorine groups are on opposite faces of the ring.
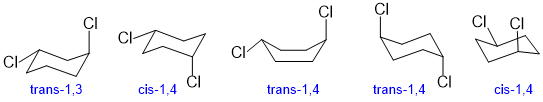
Qu30:
The torsional angle between the halogen atoms is 60 degrees in this gauche conformation.
Qu31:
The structures are isomeric (same MF) but what type of isomer are they? Comparing the drawings or using models allows one to determine that the two structures are not drawn identically and have the same components (e.g. 2 methyl groups, 2 Cl and 2 H attached to 2 other C) connected in the same order...a closer inspection shows a staggered Newman projection with (R,S) configuration (i.e. meso) and the wedge hash eclipsed and also (R,S). Therefore, they are best described as conformational isomers.
Qu32:
The most stable conformation of trans-1-ethyl-3-methylcyclohexane will be a chair conformation with the larger ethyl group equatorial and an axial methyl group.
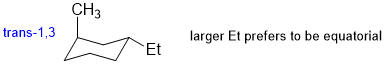
Qu33:
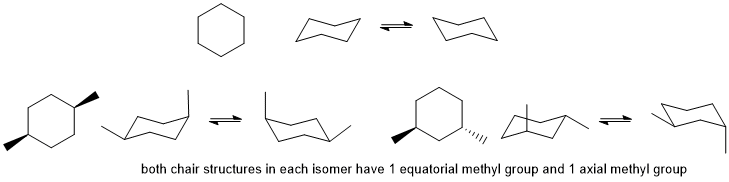
In order for the chair conformations to be of equal energy (e.g. cyclohexane itself) and if substituted then there will need to be two groups that (1) need to be the same and (2) need to be one equatorial and one axial.
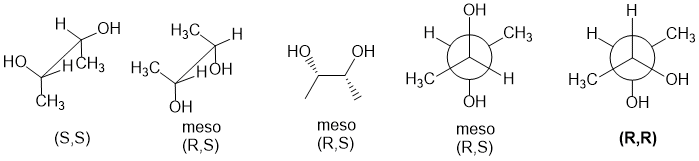
Qu34:
Use the Cahn-Ingold-Prelog rules to assign priorities to determine R/S configurations. You should be able to quickly spot the meso configurations which are (R,S) and can therefore ruled out (meso requires internal mirror plane or inversion center). This only leaves two structures that would need to be assigned.
SPECTROSCOPY:
Use any IR information to get the
functional groups. Use the H-NMR
to get the number of types of H, how many of each type from the
integral
and what they are next to from the coupling patterns. Chemical shifts
should
tell you if the group is near -O- or maybe C=O groups etc.
| Qu 35: |
IR shows a carbonyl (i.e. C=O) at 1718 cm-1 which is typical of a ketone. The C-NMR shows 4 C types including a peak for the C=O at 209 ppm suggests an aldehyde or ketone. The H-NMR has 3 types of H. The 3H triplet at 1.1 ppm,
suggests a -CH3 group next to a CH2, the 3H singlet at 2.1 ppm suggests a -CH3 group and the 2H quartet at 2.4ppm a CH2 that has 3H neighbours, i.e. an ethyl group. These pieces of data are consistent with butan-2-one. |
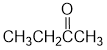 |
| Qu36: |
IR shows a carbonyl (i.e. C=O) at 1741 cm-1 which is high for a ketone. The C-NMR shows 4 C types including a peak for the C=O at 175 ppm that suggests a carboxylic acid derivative. The H-NMR has 3 types of H. The 3H triplet at 1.2 ppm,
suggests a -CH3 group next to a CH2, the 2H quartet at 2.2 ppm suggests a CH2 that has 3H neighbours, i.e. an ethyl group. The 3H singlet at 3.7 ppm,
suggests a deshielded -CH3 group without C-H neighbours such as possibly -OCH3. These pieces of data are consistent with the ester methyl propanoate. |
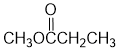 |
| Qu37: |
IR shows a carbonyl (i.e. C=O) at 1716 cm-1 which is typical for a ketone as is the C-NMR peak at 212 ppm. The H-NMR has 2 types of H. The 3H triplet at 1.1 ppm,
suggests a -CH3 group next to a CH2, and the 2H quartet at 2.4ppm suggests a CH2 that has 3H neighbours, i.e. an ethyl group. Remember that integration only gives empirical ratios. These pieces of data are consistent with pentan-3-one. |
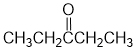 |
| Qu38: |
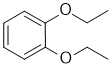
No major IR bands are reported. The C-NMR has 5 types of C including 3 in the sp2 region. The H-NMR has 3 types of H. The 3H triplet at 1.4 ppm,
suggests a -CH3 group next to a CH2 and the 2H quartet at 4.0 ppm a deshielded CH2 next to a -CH3 i.e. an ethyl group, possibly -OEt. Given the structure choices, the 2H multiplet at 6.9ppm is ArH and we need to remember that integration only gives empirical ratios.
With the 3 ArC in the C-NMR, we have ortho-diethoxybenzene. |
 |
| Qu39: |
IR shows a carbonyl (i.e. C=O) at 1743 cm-1 which is high for a ketone. The C-NMR shows 4 C types including a peak for the C=O at 171 ppm that suggests a carboxylic acid derivative. The H-NMR has 3 types of H. The 3H triplet at 1.3 ppm,
suggests a -CH3 group next to a CH2. The 3H singlet at 2.0 ppm,
suggests a -CH3 group without C-H neighbours and the 2H quartet at 4.1 ppm suggests a deshielded CH2 that has 3H neighbours, i.e. an ethyl group, possibly -OEt. These pieces of data are consistent with the ester ethyl ethanoate. |
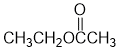 |
| Qu40: |
The presence of an -OH is shown in the IR by the broad absorption at 2700-3300 cm-1 and in the H-NMR (at 11.5 ppm, singlet that is exchangeable) : this information hints at a carboxylic acid, note the IR at 1712 cm-1 supports a C=O. The C-NMR has 4 types of C including the C=O at 181ppm. The H-NMR has 4 types of H. The 3H triplet at 1.0 ppm,
suggests a -CH3 group next to a CH2, the 2H sextet at 1.7 ppm a CH2 next to 5H (i.e. 1 x -CH3 and 1 x CH2 groups) and the 2H triplet at 2.3pmm suggests a slightly deshielded CH2 next to another CH2. These pieces are consistent with butanoic acid. |
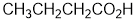 |