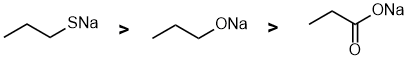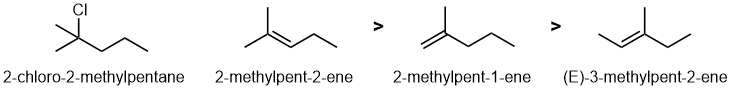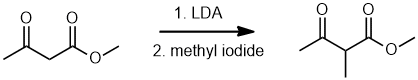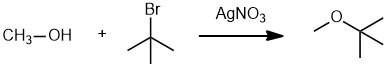Chem 351 Final Fall 2021
Here is an post-mortem analysis / "how to" for
the FINAL. The questions are split by the sections. At the start of each section
are a few suggestions of what to look for or how to tackle the question type.
RELATIVE PROPERTIES:
Identify the controlling feature,
which is not always as obvious as
it may appear. Look for two pairs of similar systems to compare that
have
minimal differences in structure.
| Qu 1: |
A question about stability carbocations. Remember that the order of stability is tertiary > secondary > primary > methyl and that allylic carbocations are further stabilised by resonance...but vinyl cations are less stable than primary. Here we have a tertiary allylic > secondary allylic > vinyl |
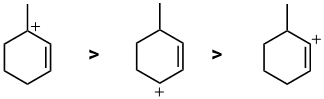 |
| Qu 2: |
Here we have 3 ionic compunds, where the heteroatom in the organic part is -ve. We have the conjugate bases of an alcohol (alkoxide), a carboxylic acid (carboxylate) and a thiol (thiolate). If we compare the alkoxide and the carboxylate (both O-) then the resonance delocalisation in the carboxylate stabilises the -ve charge and makes it less nucleophilic. If we compare the alkoxide and the thiolate (O- vs S-) then the large S atom will be more polarisable and more nucleophilic. |
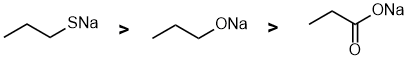 |
| Qu 3: |
First identify the reaction... SN2....(good Nu in polar aprotic solvent) which will tend to be controlled by the degree of substitution where the general trend is that primary > secondary > tertiary. We don't get simple SN2 at vinyl centers. |
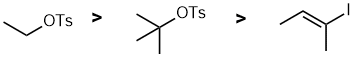 |
Qu 4: |
First draw out the structures from the names and the identify the reaction, and the reaction conditions imply alcohol dehydration.... which will tend to be E1 and therefore the stability of the intermediate carbocation. Recall that for C+ stability tertiary > secondary > primary (due to e donating properties of alkyl groups). Alkyl bromides need base to eliminate. |
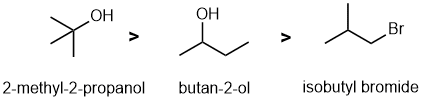 |
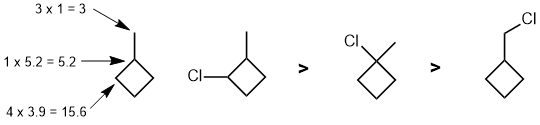
Qu 5: |
Radical chlorination of the cycloalkane (draw it) will be controlled by (i) the relative reactivity of each location, Ri and (ii) the number of H at each position, Hi
For chlorination, the reaction is controlled by a combination of the relative reactivity of the radical formed and the number of H that yield that radical. The relative reactivity of tertiary : secondary : primary systems are 5.2 : 3.9 : 1. The required calculations are outlined on the figure on the starting material. |
 |
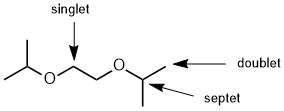
Qu 6: |
The number of lines = multiplicity (i.e. coupling) of the signals for each of the positions indicated is determined by the number of neighbours (n) that are of a different type and the n+1 rule. Here we see 7 lines (6 neighbours), a singlet and a doublet (1 neighbours). You need to remember that one doesn't observe coupling between H of the same type. |
 |
Qu 7: |
First identify the reaction...dehydrohalogenation of alkyl halides. Draw the starting material and the products, then consider the alkenes based on 1,2-elimination following Zaitsev's rule (formation of the more stable, more highly substituted alkene) using HO- Note that the E2 1,2-elimination defines the C=C location and that one of the alkenes can't be formed from this alkyl halide ! |
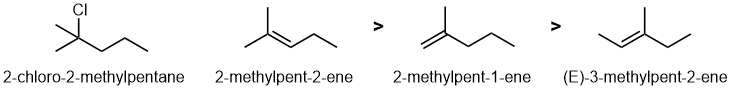 |
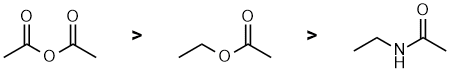
| Qu 8: |
The IR stretching frequency of a C=O is governed by the nature of the attached groups in the specific functional group. A ketone is the base / typical value at 1715 cm-1. Esters are a little higher (about 1735 cm-1) due to the increased double bond character due to the electronegativity of the O, while the anhydride in much higher (1810 & 1760) due to the electron withdrawing character of a carbonyl group. |
 |
MOLECULAR PROPERTIES
No real method here, really just do you
know various aspects of molecular structure / reactions, applied to each of the questions.
| Qu9: |
The reaction is SN1 (relates to SN laboratory experiment). Given that both bromides, the LG is the same. So the rates of reaction will depend on the stability of the carbocations involved. While both would lead to primary carbocations, the one with the benzene rings is a resonance stabilised carbocation which makes it easier to form and hence a faster reaction (v1 = B, v2 = C) |
| Qu10: |
The reactions are SN reactions. Pathway II is viable while I is not because simple SN reactions don't typically occur at sp2 C so it's the electrophile that is of concern. (v1 = D, v2 = E) |
| Qu11: |
Alanine is an amino acid, it has an amine group and a carboxylic acid group. Amine are basic (pKa about 10) and carboxylic acids are acidic (pKa about 5), so, in solution, the carboxylic acid is acidic enough to donate a proton to the amine. (v1 = D, v2 = E) |
| Qu12: |
Elimination of alkyl halides is typically E2 and there is a preference for the C-H and C-LG bonds in the H-C-C-LG to be anti (180 degrees). On a substituted cyclohexane that can dictate the regiochemistry of the elimination. One needs to draw the reactive conformation of the subsituted cyclohexane where the Br LG is in an axial position to check the locations of the reactive H atoms on the adjacent C atom. In this case, when the Br is axial, so is the methyl group and this forces an anti-Zaitsev elimination (i.e. gives the less highly substituted alkene). (v1 = D, v2 = E) |
| Qu13: |
The more nucleophilic system will have the more -ve charge on the reactive C atom. The oxygen in the carbonyl group is more electronegative than C so that means the O- contributor is more important and hence it's less reactive (v1 = A, v2 = B) |
| Qu14: |
In order to dissolve in NaOH but not NaHCO3 we are looking for phenols but not carboxylic acids (dissolve in both) (v1 =CE, v2 = BD) |
|
REACTIONS:
Three types of questions.
For those
with starting materials work from
the starting materials towards the products using the reagents to "see"
what product to look for.
For those with the product work
backwards....
looking at the functional groups in the products to think about how you
may have got there.
For those wanting reagents look at
the functional groups in the
starting material and products
to try to determine what may have happened. Look at the reagents in
each
option to see what effect they would have on the SM....
| Qu15: |
We should work backwards.... Looking at the reagents used and the structure of the product, the methoxy ether has been formed by a nucleophilic substitution (SN2) of a tosylate which would have been formed from an alcohol. The SN2 will cause an inversion of stereochemistry, so we need the alcohol with the opposite configuration at the -OH center (same configuration at the isopropyl group). (v1 = B, v2 = C) |
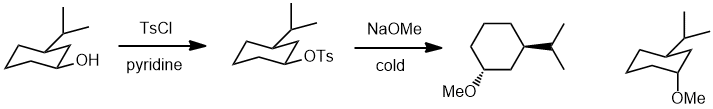 |
| Qu16: |
We should work forwards and review the transformation by comparing starting material and product. Since we are starting with an alkane, we will need to do a radical halogenation to get a reactive functional group. The halide is then been trasformed into an alcohol (SN1) and there has been a change in the skeleton which means we must have had a carbocation rearrangement during an SN1 reaction. Radical chlorination is going to tend to give a large % of primary chlorides, while bromination will give a high yield of a secondary bromide. The bromide will undergo an SN1 with aq. AgNO3 (recall the SN lab expt) to give the required rearranged alcohol. (v1 = E, v2 = A) |
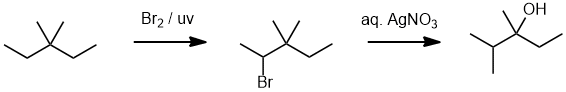 |
| Qu17: |
We should work forwards... the first reaction is an SN2 involving the conversion of the alcohol to the alkyl halide using thionyl chloride via a nucleophilic substitution and then an anti-Zaitsev E2
elimination (due to the use of the strong but bulky base) with the loss of the better leaving group (the chloride) to give the less highly substituted alkene. (v1 = E, v2 = A) |
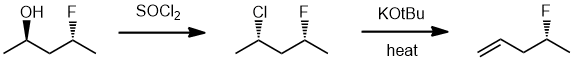 |
| Qu18: |
We should work forwards... LDA (lithium diisopropyl amide, i.e. LiN(iPr)2 ) is a strong base and will remove the most acidic H from the keto-ester starting material, that's the H in the "active methylene" between the two C=O groups. This forms a resonance stabilised "enolate" which is just a C nucleophile that then undergoes an SN2 reaction with the alkyl iodide to add the methyl group to the central C. (v1 = C, v2 = D)
|
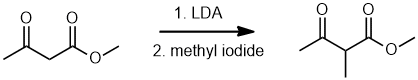 |
| Qu19: |
We should work backwards... we are trying to make an ether using AgNO3 (recall the SN lab expt) via an SN1 reaction. Ethers can be made by the reaction of an alcohol and an alkyl halide. Since the AgNO3 reacts with an alkyl halide to make a carbocation we need an alkyl halide and it is best if it yields a more stable carbocation. (v1 = C, v2 = D)
|
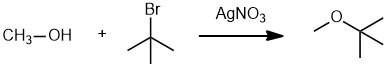
|
| Qu20: |
We should work forwards...we have an alcohol being converted into an alkene : a dehydration, an elimination. It the more highly substituted alkene (Zaitsev's rule) so that all means simple acid catalysed dehydration is all that is required. (v1 = A v2 = C) |
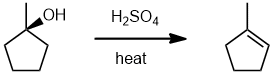 |
| Qu21: |
We can work forwards... the alcohol is being reacted with the acid HBr, which will convert the alcohol via a nucleophilic substitution to an alkyl bromide (SN1) which means a carbocation will be formed and we should consider the possibility of a rearrangement. In this case, the protonation of the R-OH will form the better LG that then leaves to give the secondary C+ which undergoes a 1,2-hydride shift to the more stable tertiary C+ and hence gives the tertiary bromide. (v1 = E, v2 = D) |
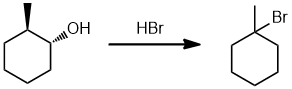 |
CONFORMATIONAL ANALYSIS:
Understanding the terminology and the
energies involved in
conformational
analysis.
Qu 22:
First, draw the named structure, then to help, count C atoms (6 total, 4 in the longest chain) and check the arrangement of the methyl groups (one on C2 and C3 of the main C4 chain) when you match it to the Newman projection. (v1 = CE, v2 = AD)
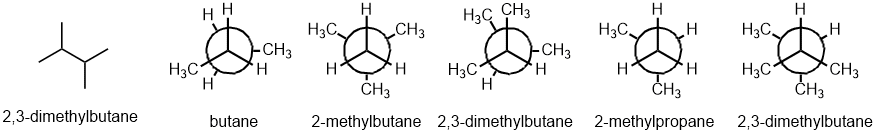
Qu 23:
The torsional angle between the equatorial methyl group and the C-C bond in the ring is 180 degrees in this chair conformation of methylcyclohexane. (v1 = AB, v2 = A)
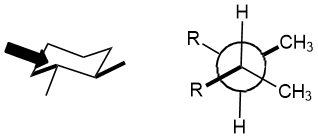
Qu 24:
The relative position of the two methyl groups are trans (they are on opposite faces of the ring). Equatorial is not a relative position. (v1 = D, v2 = E)
Qu25:
These two structures are not isomers as they have different molecular formulae (C7H16 and C6H14) (v1 =AB, v2 = D)
Qu26:
The most stable conformation of cis-1-ethyl-4-methylcyclohexane will be a chair conformation with the larger ethyl group equatorial and an axial methyl group. (v1 = CD, v2 = DE)

Qu27:
We have a staggered conformation (so rule out the two eclipsed Newman projections). Look for the stagggered conformation where the C-Cl bond is between the -F and -CH3 and anti to the C-H. (v1 = D, v2 = E)
Qu28:
Torsional strain occurs in eclipsed conformations. Cyclohexane in the chair conformation is all staggered and it therefore has minimal torsional strain. (v1 =D, v2 = C)
SPECTROSCOPY:
Use any IR information to get the
functional groups. Use the H-NMR
to get the number of types of H, how many of each type from the
integral
and what they are next to from the coupling patterns. Chemical shifts
should
tell you if the group is near -O- or maybe C=O groups etc.
| Qu29: |
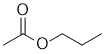
IR shows a carbonyl (i.e. C=O) at 1745 cm-1 which is high for a ketone. The C-NMR shows 5 C types including a peak for the C=O at 171 ppm suggests a carboxylic acid or derivative. The H-NMR has 4 types of H. The 3H triplet at 1.0 ppm,
suggests a -CH3 group next to a CH2, the 2H sextet at 1.6 ppm suggests a CH2 group with 5 neighbours, and the 3H singlet at 2.0 ppm a CH3 that has no neighbours and the 2H triplet at 4.1 ppm suggests a -CH2 group next to a -CH3,. These pieces of data are consistent with an ester but with a methyl group adjacent to the C=O i.e. propyl ethanoate. |
 |
| Qu30: |
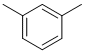
IR has no carbonyl (i.e. C=O) near 1715 cm-1 but shows C=C near 1600 cm-1. The C-NMR shows 5 C types including 4 ArC 110-170 ppm that suggests a substituted benzene. The H-NMR has at least 2 types of H (note that all the ArH are showing as a multiplet). The 3H singlet at 2.3 ppm,
is consistent with a -CH3 group attached to the benzene ring. Checking the integration shows a 3:2 ratio which implies there needs to be 2 methyl groups to create a disubstituted benzene (i.e. 6:4). Given the number of ArC, it must be meta substituted, i.e. 1,3-dimethylbenzene. |
 |
| Qu31: |
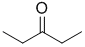
IR shows a carbonyl (i.e. C=O) at 1720 cm-1 which is typical for a ketone as is the C-NMR peak at 212 ppm. The H-NMR has 2 types of H. The 3H triplet at 1.1 ppm,
suggests a -CH3 group next to a CH2, and the 2H quartet at 2.4ppm suggests a CH2 that has 3H neighbours, i.e. an ethyl group. Remember that integration only gives empirical ratios. These pieces of data are consistent with pentan-3-one. |
 |
| Qu32: |
IR has no carbonyl (i.e. C=O) near 1715 cm-1 but shows C=C near 1500 cm-1. The C-NMR shows 5 C types including 4 ArC 110-170 ppm that suggests a substituted benzene. The H-NMR has at least 3 types of H (note that all the ArH are showing as a multiplet). The 3H triplet at 1.2 ppm,
is consistent with a -CH3 group next to a CH2, the 2H quartet at 2.6 ppm a CH2 next to a -CH3 group, so it looks like an ethyl group.. Checking the integration shows a 3:2:5 ratio which is consistent with a mono-substituted benzene, i.e. ethylbenzene. |
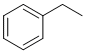 |
| Qu33: |
IR shows a carbonyl (i.e. C=O) at 1717 cm-1 which is typical for a ketone as is the C-NMR peak at 209 ppm. The C-NMR shows 5 C types including a peak for the C=O at 209 ppm. The H-NMR has 4 types of H. The 3H triplet at 0.9 ppm,
suggests a -CH3 group next to a CH2. The 2H sextet at 1.6 ppm,
suggests a -CH2 group with 5 H neighbours, the 3H singlet at 2.1 ppm,
suggests a -CH3 group with 0 neighbours (slightly deshielded) and the 2H triplet at 2.4 ppm suggests a slightly deshielded CH2 that has 2H neighbours, together this suggests i.e. a methyl group and a propyl group. These pieces of data are consistent with the ketone, pentan-2-one. |
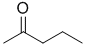 |
| Qu34: |
IR shows a carbonyl (i.e. C=O) at 1715 cm-1 which is typical for a ketone as is the C-NMR peak at 212 ppm. The C-NMR shows 5 C types including a peak for the C=O at 212 ppm. The H-NMR has 3 types of H. The 1H pentet at 1.7 ppm,
suggests a -CH group next to 4H, the 2H pentet at 1.9 ppm,
suggests a -CH2 group with 4 H neighbours, and the 2H triplet at 2.3 ppm suggests a slightly deshielded CH2 that has 2H neighbours. These pieces of data are consistent with the ketone, cyclohexanone. |
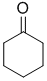 |