Note that no other reagents are needed in order to complete any of these sequences, you should only be using what is there.
Part A:
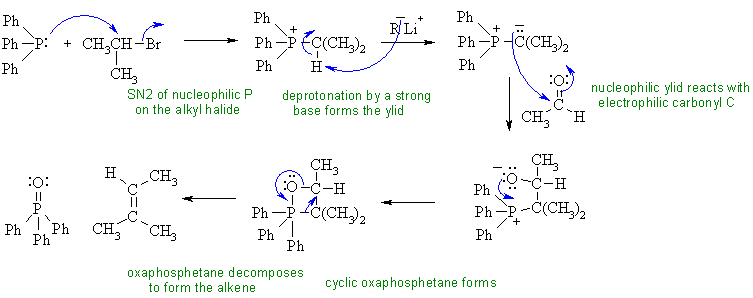
i. This is an example of the Wittig reaction.

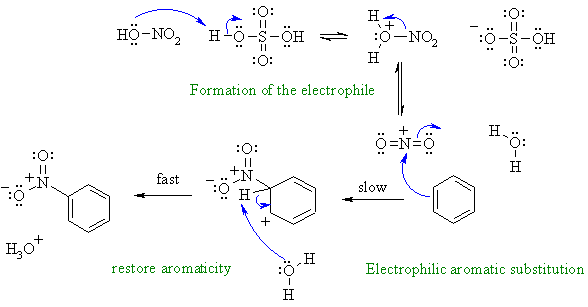
ii. Nitration of an aromatic via an electrophilic aromatic substitution. First, need to show the formation of the reactive electrophile from the nitric acid / sulfuric acid mixture.
Part B:
i. This is an example of an electrohilic aromatic substitution where the electrophile is D+. The large alkyl group directs mainly para-.
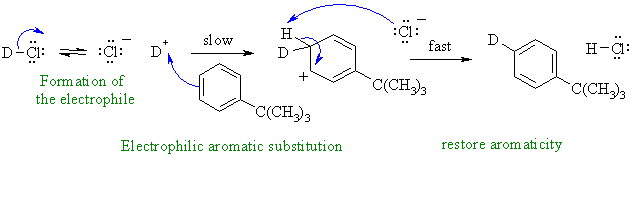
Common errors in this question were not recognising the correct reaction type and introducing -Cl as the substituent rather than -D or thinking the reaction was radical in character.
ii. An example of an electrophilic addition to an alkene but where the Nu is a nitrogen of a nitrile. The resulting cation is then undergoes hydrolysis and then tautomerises to the product. While it appears "complex" the principles are quite simple....

Common general errors: (1) Ignoring reaction conditions i.e. effect of acidic or basic environment (2) poorly drawn arrows, e.g. not starting at the Nu site (3) backwards arrows (4) wrong use of arrows e.g. resonance (5) not showing formal charges (6) missing arrows esp. when adding or removing H+ (7) compressing several mechanistic steps into a single step.