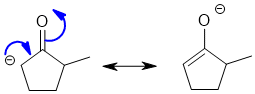353 MT Winter 2023
WORK IN PROGRESS (images to create and add, explanations to be reviewed).

Here is an post-mortem analysis / "how to" for the MT. The questions are split by the sections. At the start of each section are a few suggestions of what to look for or how to tackle the question type.
RELATIVE PROPERTIES:
Identify the controlling feature, which is not
always as obvious as it may appear. Look for two pairs of similar systems to compare
that have minimal differences in structure. If a compound is named, draw it out.
If a reaction is involved, identify the type of reaction and then what the controlling
factors are.
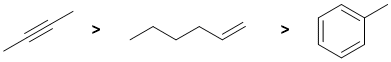
Qu1:
The reaction is a catalytic hydrogenation. The reaction is fastest for the weakest pi bond and that is found in an alkyne, so the alkyne is the most reactive. In contrast, the C=C in arenes are less reactive (due to conjugation and aromatic stability) than those in alkenes.
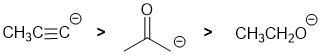
Qu2:
We have the conjugate bases, so use the approx pKas of the acids: there is an terminal alkyne, so the CH is likely about 25, a ketone = 20 and an and an alcohol OH = 15. The electronegativity of the atom the acidic H is attached to is a key factor (OH vs CH), the atom hybridisation (alkyne) and resonance stabilisation (ketone). Remember the question wants in basicity order (higher acid pKa = stronger base).
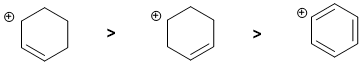
Qu3:
Carbocation stability.... Look at the C atom bearing the charge and what's attached to it. We have a secondary allylic carbocation, a simple secondary carbocation and a phenyl carbocation. The simple secondary allylic carbocation is the most stable (remember the extra resonance stabilisation). Then the secondary carbocation is more stable than the phenyl carbocation.
Qu4:
All the bonds are C-C bonds. The factors involved are the hybridisation of the C atoms involved. C-C bonds involving two sp2 C atoms are shorter than two sp3 C atoms (due to higher s character).
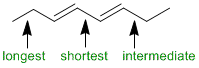
Qu5:
Identify the chirality centers (*) each of which can be R or S so here we have 4 configurations, 3 (one is meso), and 1 (not chiral). Remember that the maximum number of configurational isomers is 2n where "n" is the number of stereocenters.
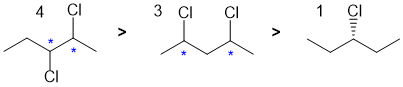
Qu6:
First, draw the named starting material. Hydroboration / oxidation of alkenes tends to give the less substituted alcohol (which is the anti-Markovnikov product). Therefore the major product will be the anti-Markovnikov primary alcohol, but there will be some Markovnikov secondary alcohol formed. Since there is no carbocation formed in this reaction, we will not get the tertiary alcohol.
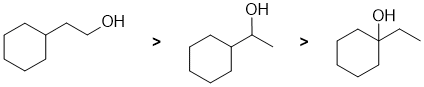
Qu7:
How many H atoms are there on the C atoms that are adjacent to the C=O group ? (alpha H are highlighted in blue below)
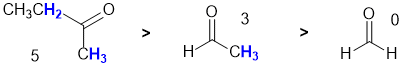
Qu8:
All about specific rotation. Looking at the three structures, we need to use the Cahn-Ingold-Prelog rules to assign the configurations. One (wedge hash diagram) is (R,R), one (Newman projection) is (S,S) and the other (Fisher projection) is (R,S), a meso compound and therefore is optically inactive and has by definition a specific rotation = zero.
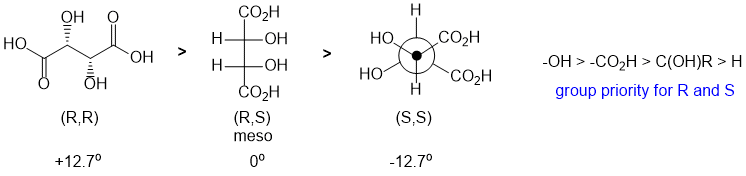
Qu9:
Alkenes undergo electrophilic addition. In the reactions the rate is controlled by the rate of carbocation formation formed when the acid protonates (i.e. adds H+ to the C=C). In this case, this means the the stronger the acid, the faster the reaction. HCl has a pKa = -7, CH3CO2H = 5 and H3O+ = 0. Lower the pKa the stronger the acid so:
HCl > H3O+ > CH3CO2H
Qu10:
Hydroboration / oxidation of alkenes tends to give the less substituted alcohol (which is the anti-Markovnikov product). Alkan-2-ol means R-CH(OH)-CH3. One alkene is C4 and symmetrical and can only give butan-2-ol, the other alkene will give a mixture of pentan-2-ol and pentan-3-ol. Alkynes undergo hydroboration / oxidation to give aldehydes or ketones not alcohols.
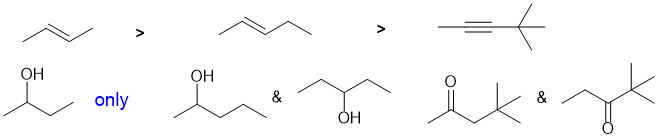
STARTING MATERIALS, REAGENTS AND PRODUCTS:
If you are trying to find the product, then
you should probably just work forwards through the sequence of reactions.
If you are looking for the starting material,
then working backwards is probably the best way to go....
Basically depends on the need to know and identify
the reactions, this is often triggered by looking at the functional groups in
the molecules.
Qu11:
Working forwards : hydration of terminal alkynes gives methyl ketones as the major product (+ve charge preferred on the secondary rather than the primary carbon).
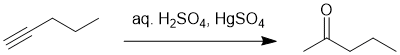
Qu12:
Working forwards : We need to eliminate the alcohol but get the less highly substituted alkene (anti-Zaitsev elimination). Simple acid catalysed dehydration of an alcohol will give the Zaitsev product (hex-2-ene). Therefore use SN2 reaction to convert the alcohol to the alkyl halide then eliminate the alkyl halide with a bulky base.

Qu13:
Working forwards : form the 1,2-dibromide via an anti addition then double elimination of an alkyl halide (Zaitsev elimination) forms the conjugated diene. Then heating the conjugated diene with ethene suggests a Diels-Alder reaction followed catalytic hydrogenation of the alkene to the alkane.

Qu14:
Working backwards : in order to have formed a 1,1-dibromide using HBr (normal conditions), then one needs to have started with an alkyne and the terminal alkyne will tend to be the more selective : hydrohalogenation of an alkyne.
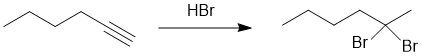
Qu15:
Working forwards : simple hydration of internal alkynes gives a 50:50 mixture of ketones, while hydroboration / oxidation of an alkyne can be more selective at giving the C=O on the less hindered side, especially if one uses 9-BBN over BH3 (9-BBN is more hindered and therefore the steric effect is enhanced 96% > 75%).
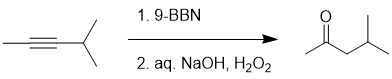
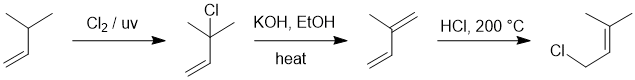
Qu16:
Working forwards :
allylic radical substitution adds the Cl, then E2 elimination to give the favoured (stability) conjugated diene. Step 3 is thermodynamically controlled 1,4- addition.
Qu17:
Working forwards : terminal alkyne alkylation = deprotonation of a terminal alkyne to make a good nucleophile followed by an SN2 to yield the internal alkyne.
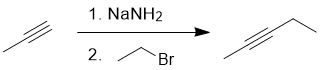
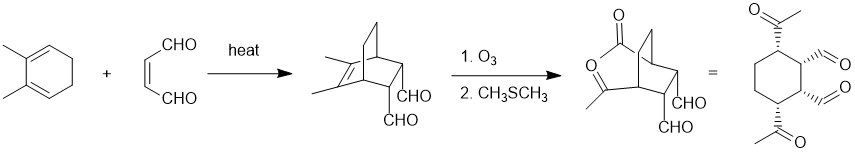
Qu18:
Working forwards : a Diels-Alder reaction of a conjugated diene being heated with an alkene with electron withdrawing groups (the dienophile) to give the favoured endo product. This is followed by ozonolysis of the alkene with a reductive work up to converted the substituted alkene into the methyl ketones. Be careful with the relative positions of the groups!
REGIO- and STEREOCHEMISTRY:
How well do you know your reagents ? Look
at what has actually happened in terms of the reaction functional group transformation
and then first look for any regiochemical issues then finally the stereochemistry
last (it's the hardest to sort out). In cases where more than one product
is formed in equal amounts (e.g. the enantiomers), then both must be selected
for full marks, part marks are given when only one of the pair is selected.
Advice : in each case draw the starting material in the
conformation in which is reacts or the product in the conformation in which
it is initially formed using wedge-hash diagrams. It is a good idea to draw
the materials in such a way that the new bonds are in the plane of the page.
Once you have drawn the materials it may also be good for you to use model kits
for these questions too. Once you have drawn the materials in this way,
you may need to consider rotations around sigma bonds to make your answer match
the options. An alternative approach could be to assign configurations
to your drawn answer to compare them with the options - this can be slow and
prone to error.
Qu19:
Working backwards : Bromine adds to alkenes to give 1,2-dibromides via an anti addition. Redraw the product in the conformation in which it formed (e.g. rotate the front C in the Newman projection 120 degrees clockwise to put the Br at 180 degrees and reveal that the alkene will need to be trans.
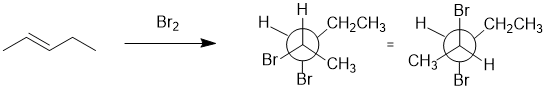
Qu20:
Working forwards : potassium permanganate reacts with alkenes to give cis-1,2-diols via a syn addition. This means that there will be 2 -OH groups added on adjacent C and they will be on the same face of the ring.
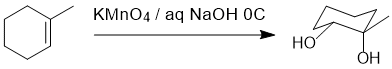
Qu21:
Working forwards : dissolving metal reduction to the trans-alkene. The peracid forms the epoxide (syn addition, alkene stereochemistry same as epoxide stereochemistry, trans) followed by ring opening under basic conditions (strong Nu) to put the methoxy group at the least hindered end (SN2 like) with overall stereochemistry that is anti from the alkene. It's usually best to work through using wedge/hash then convert to the Fisher projection.
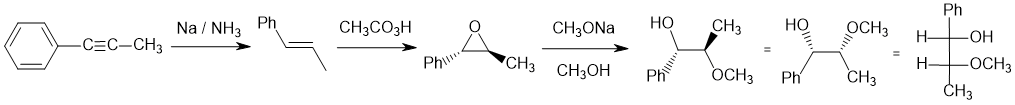
Qu22:
Working forwards : simple acid catalysed hydration of the alkene to give the Markovnikov (more substituted) alcohol.
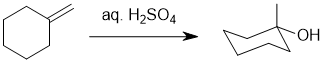
Qu23:
Working forwards : the halohydrin reacting with Na2CO3 (a weak base) to make an epoxide via an SN2 reaction so we need to make sure we review the halohydrin in the reactive conformation with the O nucleophile undergoing a backside attack to the C-Br bond so -OH and -Br anti to each other... so best to redraw the starting material with the -OH up (since the epoxides all have the O on the upper side) and the -Br anti to the O (180 degrees for SN2 backside attack). This gives the epoxide with the Ph and methyl groups trans but the configurations are defined by the starting material shown in the question.
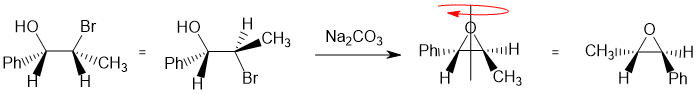
Qu24:
Working forwards : dissolving metal reduction to the trans-alkene. In step 2, we have a Simmons-Smith cyclopropanation of a C=C to make the cyclopropane (syn addition, alkene stereochemistry same as cyclopropane stereochemistry, cis).
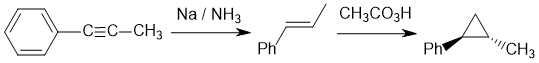
Qu25:
Working forwards : the unsymmetrical epoxide has undergone ring opening with the acetylide (a strong Nu, hence SN2 like) at the less hindered end. Hydration of the terminal alkyne then gives the methyl ketone.
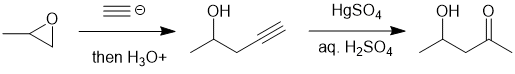
PI SYSTEMS:
All about knowing properties of pi systems such as resonance, structure, bonding, shapes, and terminologies etc.
Qu26:
Conjugation requires an extended pi system (at least 3 atoms) where the pi systems can interact
(parallel orbitals, not perpendicular). The image below has highlighted the p orbitals (blue circles for top down view of vertical p orbitals.
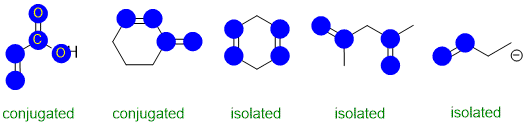
Qu27:
Resonance contributors are derived via the delocalisation of the pi electrons across the pi system and can be derived by pushing curly arrows. Use the rules for recognising resonance structures.
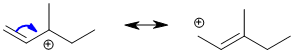
Qu28:
We are looking at a Diels-Alder reaction. Methyl propenoate is the dienophile, so we need to use a conjugated diene. Electron donating groups and being locked in the reactive s-cis conformation make 1,3-cyclohexadiene a very good diene.
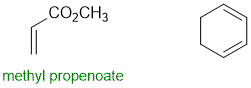
Qu29:
The lowest energy is the most stable compound. The key issues are (1) trans isomer are more stable than cis isomers (steric effects), (2) s-trans is typically more stable than s-cis. Therefore the least stable isomer is the s-trans-trans,trans-conjugated diene.
![]()
Qu30:
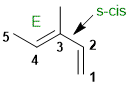
Qu31:
Organic cis / trans nomenclature is based on the stereochemistry (shape) of the chain the defines the root name.
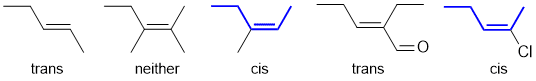
Qu32:
The addition of hypohalous acids occurs via a cyclic halonium ion.

Qu33:
The reaction is a catalytic hydrogenation. The reaction shown is H2 with Lindlar's catalyst which is a reduction of alkynes (doesn't reduce alkenes or the other functional groups)
![]()
Qu34:
Resonance contributors are derived via the delocalisation of the pi electrons across the pi system and can be derived by pushing curly arrows. Use the rules for recognising resonance structures.