353 MT Winter 2024
Here is an post-mortem analysis / "how to" for the MT. The questions are split by the sections. At the start of each section are a few suggestions of what to look for or how to tackle the question type.
RELATIVE PROPERTIES:
Identify the controlling feature, which is not
always as obvious as it may appear. Look for two pairs of similar systems to compare
that have minimal differences in structure. If a compound is named, draw it out.
If a reaction is involved, identify the type of reaction and then what the controlling
factors are.
Qu1:
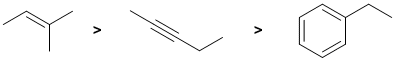
Alkenes undergo electrophilic addition. In the reactions with aq. H2SO4, the rate is controlled by the rate of carbocation formation. In this case, this means the more stable the carbocation, the faster the reaction. The simple alkene will react the faster (via a tertiary carbocation) than the alkyne since it would require the much less favourable termolecular pathway to avoid the formation of the very unfavourable vinyl carbocations. The ease of carbocation formation dictates the rate of reaction. In the case of alkynes, the vinyl C+ would be too unstable and a slower termolecular mechanism occurs, so the alkyne is slower. The C=C in benzenes don't under electrophilic addition.

Qu2:
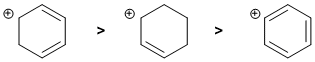
Carbocation stability.... Look at the C atom bearing the charge and what's attached to it. We have a secondary allylic carbocation, a secondary carbocation conjugated to a diene and a phenyl carbocation. More delocalisation of the positive charge will give a more stable carbocation so the diene system has 3 contributors while the allylic system 2 contributors. Phenyl carbocations aren't delocalised (think about the orbital alignments).
Qu3:
Acidity and pKas... if you know your pKas this is easy. Or think least acidic (high pKa) to most acidic (low pKa)... Ammonia has a pKa = 35, terminal alkynes = 25 and alcohols = 15 (question asks for relative pKa). The pKa values are affected by the electronegativity of the atom to which the acidic H is attached and the hybridisation of that atom (think %s character and the effect of nuclear stabilisation).
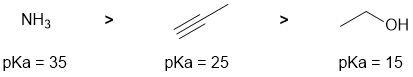
Qu4:
The reaction is
catalytic hydrogenation which reduces alkynes to alkenes and alkenes to alkanes. In contrast, the C=C in arenes are less reactive (due to conjugation and aromatic stability) than those in alkenes. This will mean that with H2 (which will naturally be present in excess, most of the alkyne starting material will be reduced to the alkane but there will be some alkene present too. The C=C in the arene will not be reduced to any significant extent so there will be minimal (essentially zero) reduction to the cyclohexane.
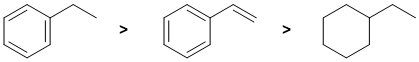
Qu5:
Draw them out and identify which C=C can be cis or trans... Both C=C in the 2,4- isomer can be cis or trans so we have 3 geometric isomers (cis,cis & cis,trans & trans,trans), the 1,3- isomer has one C=C that can be cis or trans so 2 geometric isomers and the 1,5-isomer C=C don't have cis/trans isomers.
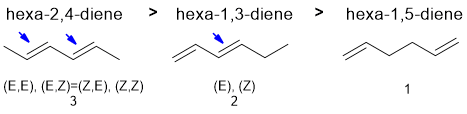
Qu6:
Draw out the named structure... that alkene reacts with MCPBA (meta-chloroperbenzoic acid, a peracid) to give an epoxide. The epoxide ring opening is under acidic conditions with a weak nucleophile which means the unsymmetrical protonated epoxide (a cationic intermediate) is preferentially opened when the ROH nucleophile attacks the more substituted C (SN1 like fashion) to give the alkoxy alcohol.

Qu7:
Draw out the named structure and it will likely help you recognise the Diels-Alder reaction of a conjugated diene being reacted with a conjugated ketone (the dienophile). The diene in a Diels-Alder reaction is more reactive if it (1) has electron donating groups and (2) favours the reactive s-cis conformation. Cyclopentadiene is a very good diene because it is locked s-cis and effectively has alkyl groups (weak electron donors) at each end of the diene unit. Of the two acyclic dienes, the cis,cis isomer is less reactive because steric effects destablise the reactive s-cis conformation which means very little of the molecule is in the reactive conformation at any one time.
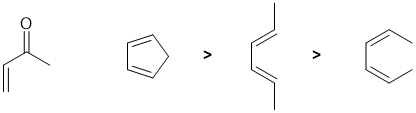
Qu8:
All about specific rotation [a]D
= a / cl when
concentration is expressed as g sample / ml solution. Just use the formula and plug in the numbers to calculate the observed rotations...
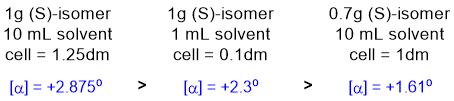
Qu9:
Draw out the named structure... the conjugated diene will undergo conjugate addition (1,4-addition = major product) of Cl2 at the higher temperature (i.e. thermodynamic control) with the direct addition (1,2-addition) being the other product.
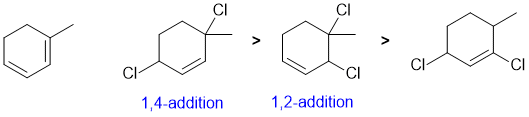
Qu10:
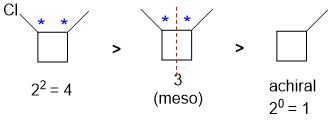
Identify the chirality centers (if any) and then the maximum number of configuration isomers possible (2n where n = number of chirality centers). Then check for meso compounds...
STARTING MATERIALS, REAGENTS AND PRODUCTS:
If you are trying to find the product, then
you should probably just work forwards through the sequence of reactions.
If you are looking for the starting material,
then working backwards is probably the best way to go....
Basically depends on the need to know and identify
the reactions, this is often triggered by looking at the functional groups in
the molecules.
Qu11:
Working forwards : potassium permanganate reacts with alkenes to give cis-1,2-diols via a syn addition followed by double acid catalysed dehydration of an alcohol will give the more stable, most substituted conjugated diene.
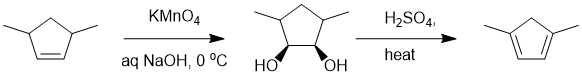
Qu12:
Working forwards : in order to have formed a 1,1-dibromide from a terminal alkyne one needs a radical addition (anti-Markovnikov) reaction : radical hydrobromination of an alkyne.
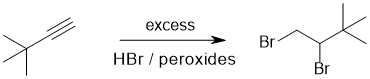
Qu13:
Working forwards : double elimination of an alkyl halide (Zaitsev elimination) forms the conjugated diene. Then heating the conjugated diene with the enal (a conjugated aldehyde is a good dienophile) indicates Diels-Alder reaction : draw out the mechanism to ensure you get the right C=C in the right locations.
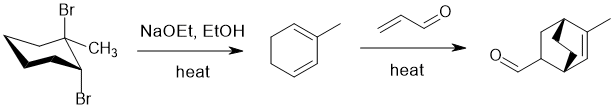
Qu14:
Working backwards : the product is an aldehyde that would be formed by the anti-Markovnikov hydroboration / oxidation of an alkyne since it will be more selective at giving the C=O on the less hindered side, especially if one uses 9-BBN over BH3 (9-BBN is more hindered and therefore the steric effect is enhanced).
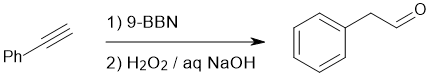
Qu15:
Working forwards : terminal alkyne alkylation = deprotonation of a terminal alkyne to make a good nucleophile followed by an SN2 to yield the symmetrical, internal alkyne. Then alkyne ozonolysis gives the carboxylic acid. Count C atoms...
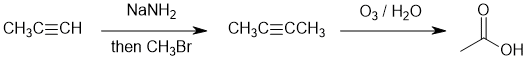
Qu16:
Working forwards : the halohydrin reacting with Na2CO3 (a weak base) to make an epoxide via an SN2 reaction. The epoxide then undergoes ring opening under basic conditions (acetylide ion = strong Nu) to introduce the alkyne group.

Qu17:
Working backwards : the product is a 1,2-diol that would have been made from an alkene. We need to analyse the stereochemistry of the product and that shows that we either need to do an anti addition to a cis-alkene or syn addtion to a trans-alkene. Only one of these options is available as a choice.
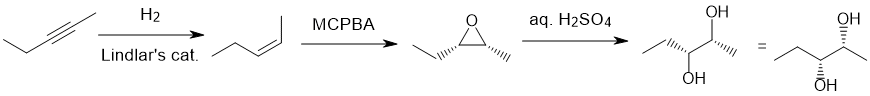
Qu18:
Working backwards : The diketone has been formed from ozonolysis with a reductive work-up of an intramolecular alkene. "Clip" the carbonyl groups back together (count C atoms carefully) to reveal the alkene required which seems to have been formed by acid catalysed dehydration of an alcohol with a rearrangement to give the tetrasubstituted alkene.
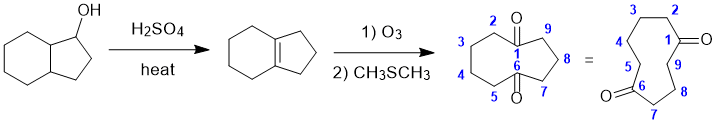
REGIO- and STEREOCHEMISTRY:
How well do you know your reagents ? Look
at what has actually happened in terms of the reaction functional group transformation
and then first look for any regiochemical issues then finally the stereochemistry
last (it's the hardest to sort out). In cases where more than one product
is formed in equal amounts (e.g. the enantiomers), then both must be selected
for full marks, part marks are given when only one of the pair is selected.
Advice : in each case draw the starting material in the
conformation in which is reacts or the product in the conformation in which
it is initially formed using wedge-hash diagrams. It is a good idea to draw
the materials in such a way that the new bonds are in the plane of the page.
Once you have drawn the materials it may also be good for you to use model kits
for these questions too. Once you have drawn the materials in this way,
you may need to consider rotations around sigma bonds to make your answer match
the options. An alternative approach could be to assign configurations
to your drawn answer to compare them with the options - this can be slow and
prone to error.
Qu19:
Working forwards : Alkenes undergo halohydration when reacted with chlorine in water via an anti addition with the -OH group at the more substituted C (reaction occurs via a cyclic chloronium ion with H2O then reacting as the nucleophile and attacking the more substituted C in an SN1 like fashion).
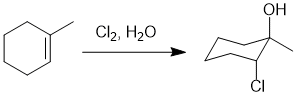
Qu20:
Working forwards : The first reaction is
catalytic hydrogenation which reduces the alkyne to the cis-alkene. This alkene reacts with the peracid via a syn-addition to give the epoxide with the same stereochemistry. The epoxide ring opening is under acidic conditions with a weak nucleophile which means the unsymmetrical protonated epoxide (a cationic intermediate) is preferentially opened when the ROH nucleophile attacks the more substituted C (SN1 like fashion) to give the alkoxy alcohol and then work out the required Fischer projection (remember to position the alkyl chain (vertical) and back into the page with all the horizontal bonds coming out towards you)

Qu21:
Working backwards : potassium permanganate reacts with alkenes to give cis-1,2-diols via a syn addition so rotate the product Newman projection into the conformation in which it is formed to reveal the stereochemistry of the alkene that is required.
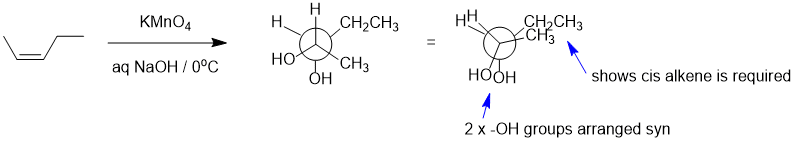
Qu22:
Working forwards : HBr, peroxides will undergo the radical addition to the alkene to give the anti-Markovnikov secondary bromide. Elimination of the alkyl bromide with a bulky base will be anti-Zaitsev to give the less highly substituted terminal alkene. Hydroboration / oxidation of alkenes tends to give the less substituted primary alcohol (which is the anti-Markovnikov product).

Qu23:
Working forwards : Cyclopenta-1,3-diene is a "classic" Diels-Alder diene and will react with dienophiles in a Diels-Alder reaction. The stereochemistry of the dienophile is preserved in the product. This is then followed by a catalytic hydrogenation of the cyclohexene to the cyclohexane.
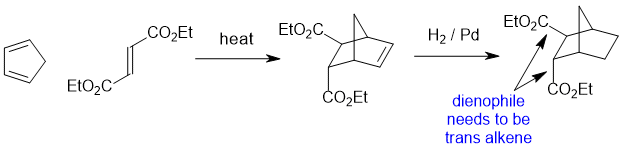
Qu24:
Working forwards : The halohydrin reacting with Na2CO3 (a weak base) to make an epoxide via an SN2 reaction so we need to make sure we review the halohydrin in the reactive conformation with the O nucleophile undergoing a backside attack to the C-Cl bond so -OH and -Cl anti to each other... that reveals that the Ph- and CH3- are trans to each other... then just rotate to the whole molecule to get the groups lined up to ensure you get the right configurations.
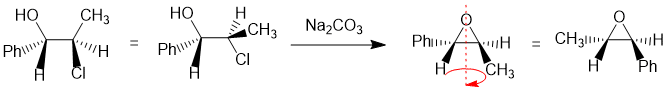
Qu25:
Working forwards : HBr will undergo the addition to the isolated diene as if it were an alkene, adding to the C=C unit that gives the more stable carbocation in accord with Markovnikov's rule.

PI SYSTEMS:
All about knowing properties of pi systems such as resonance, structure, bonding, shapes, and terminologies etc.
Qu26:
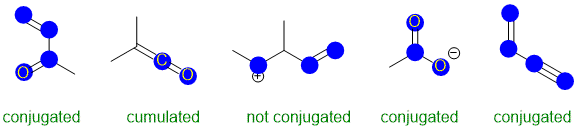
Conjugation requires an extended pi system (at least 3 atoms) where the pi systems can interact
(parallel orbitals, not perpendicular). The image below has highlighted the p orbitals (blue circles for top down view of vertical p orbitals.
Qu27:
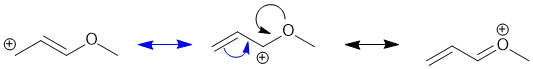
Resonance contributors are derived via the delocalisation of the pi electrons across the pi system and can be derived by pushing curly arrows (blue to the left contributor, black to the right contributor). Use the rules for recognising resonance structures.
Qu28:
We are looking at a Diels-Alder reaction.1,3-butadiene is the diene component, so we need to use a dienophile. Electron withdrawing groups (e.g. -CO2R) make better dienophiles.
Qu29:
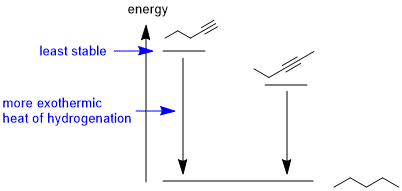
Draw the picture...The most exothermic heat of hydrogenation means we need to identify the least stable compound, hence the less highly substituted alkyne (since the second pi bond in a alkyne is weaker than an alkene pi bond).
Qu30:
s-cis (3Z)-2,3-dimethylpenta-1,3-diene :
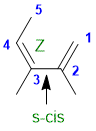
Qu31:
Allylic means on the C next to an alkene C=C.
Qu32:
The addition of halogens to alkenes occurs via a cyclic halonium ion.
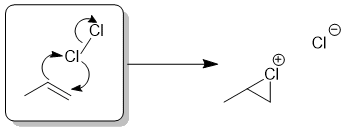
Qu33:
Na / NH3 is the dissolving metal reduction used to reduce alkynes to trans-alkenes

Qu34:
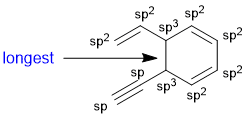
The longest CC bond will be the single bond between two sp3 C atoms.