Part A
i This reaction is the hydrolysis of an acetal.
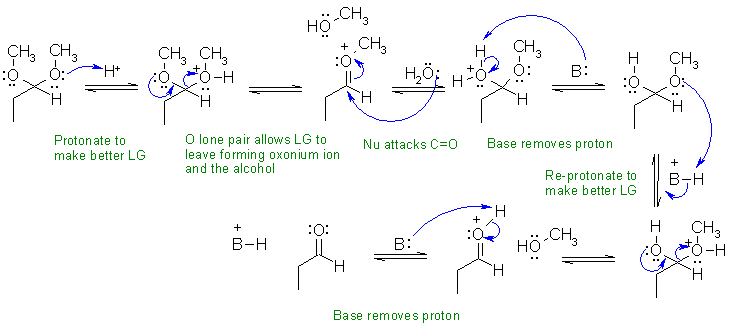
In this scheme, the base, B:, could be R-O-R, R-OH, H2O or the conjugate base of the acid catalyst
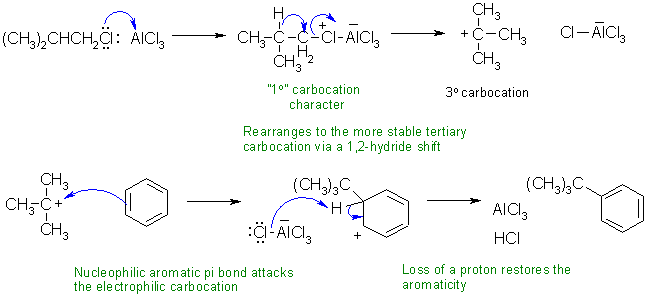
ii This is a Friedel-Crafts
alkylation of an aromatic via an electrophilic aromatic substitution
involving a carbocation
rearrangement.
iii This is an acid catalysed transesterification....
the -OH of the molecule displaces the methanol to give the cyclic ester.
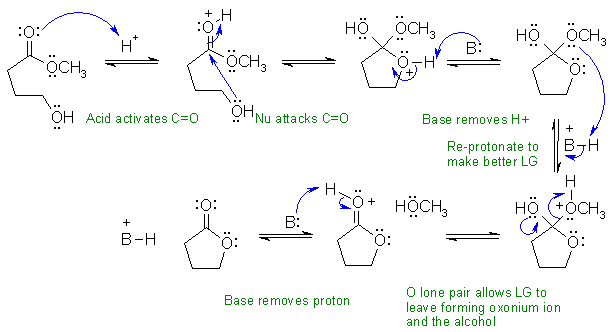
In this scheme, the base, B:, could be C=O, R-OH, or the conjugate base of the acid catalyst
Part
B
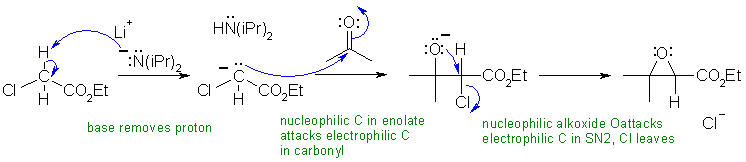
i LiN(iPr)2 is LDA, a strong base (pKa = 35),
which will form an enolate
of the ester. This enolate will then react with the ketone (similar
to the aldol
reaction). The product of this step contains an alkoxide and there is
a good leaving group (the chlorine) on the adjacent carbon so an intramolecular
Williamson
type reaction - just SN2 - happens to form a cyclic ether, the epoxide
(note : this last step is also like
using a halohydrin
to form an epoxide)
ii The starting material has a ketone and an epoxide. The product has neither of these, but on careful examination has a cyclic acetal. The key steps are therefore (a) opening of the epoxide to give the 1,2-diol then (b) reaction of the diol with the ketone to give the cyclic acetal. Numbering helps you to see how the structures relate to each other.
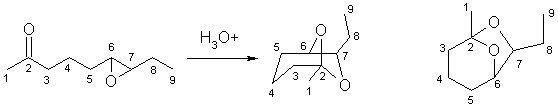
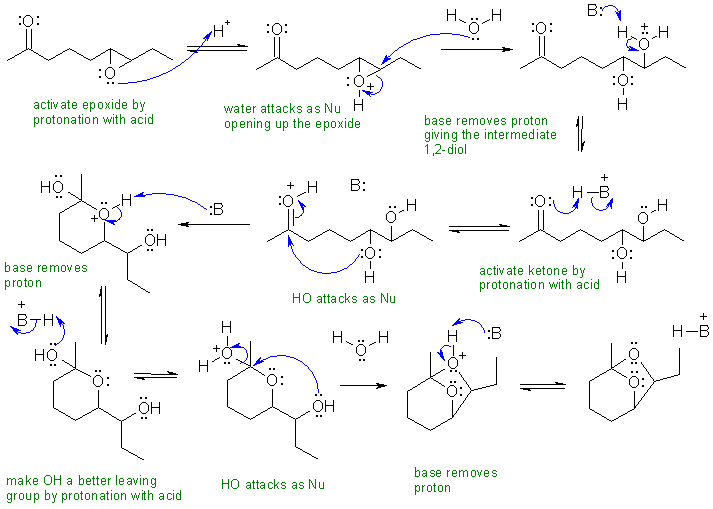
In this scheme, the base, B:, could be C=O, R-O-R, R-OH, H2O or the conjugate base of the acid catalyst
![[Chem 350 Home]](mol.gif)