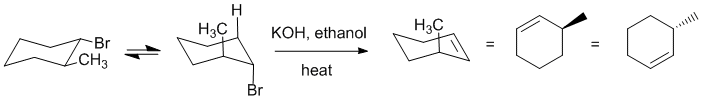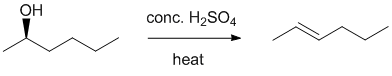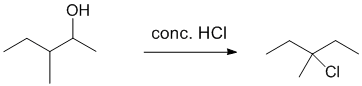Chem 351 Final Fall 2016
Here is an post-mortem analysis / "how to" for
the FINAL. The questions are split by the sections. At the start of each section
are a few suggestions of what to look for or how to tackle the question type.
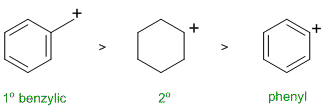
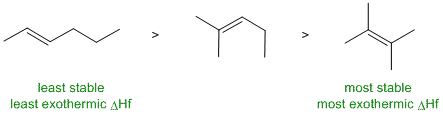
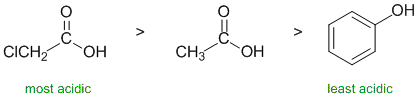
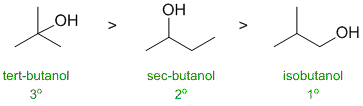
RELATIVE PROPERTIES:
Identify the controlling feature,
which is not always as obvious as
it may appear. Look for two pairs of similar systems to compare that
have
minimal differences in structure.
MOLECULAR PROPERTIES
No real method here, really just do you
know various aspects of molecular structure / reactions, applied to each of the questions.
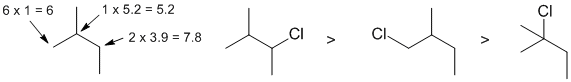
| Qu13: |
2-bromo-2-methybutane is a tertiary alkyl halide and the dominant reaction with ethoxide (EtO-) is the elimination pathway where the EtO- reacts as a strong base (only 1% of the products formed are the ether II) |
| Qu14: |
The chemical shift of the alkyne H (2-3 ppm) is due to the magnetic anisotropy of the triple bond and the position of the H atom in the shielding cone of the induced magnetic field. |
| Qu15: |
Iodide acts as a catalyst because it reacts with the alkyl chloride to make the alkyl iodide which is a better leaving group. Once the alkyl iodide reacts with the cyanide, the iodide is regenerated. |
| Qu16: |
Step 2 is an SN2 reaction (step 1 is the formation of a tosylate which makes the -OH in to a better leaving group). SN2 reactions occur via backside attack and this results in a Walden inversion. |
| Qu17: |
The bond vibration is modeled by Hooke's law and depends on i. bond strength and ii. the masses attached at each end. Both are CH bonds so the same masses are involved. The higher s character makes the sp C-H bond stronger than the sp3 C-H. |
| Qu18: |
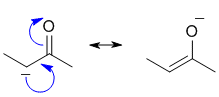
Push the curly arrows! Only 1 of the choices is a resonance contributor (check overall charge and changes to sigma bonds).
 |
REACTIONS:
Three types of questions.
For those
with starting materials work from
the starting materials towards the products using the reagents to "see"
what product to look for.
For those with the product work
backwards....
looking at the functional groups in the products to think about how you
may have got there.
For those wanting reagents look at
the functional groups in the
starting material and products
to try to determine what may have happened. Look at the reagents in
each
option to see what effect they would have on the SM....
| Qu19: |
We should work backwards...Sodium methoxide can react via a nucleophilic substitution with an alkyl halide to give a methyl ether. The alkyl halide is formed via a radical bromination of an sp3 C. Remember to count C atoms. |
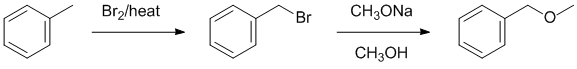 |
| Qu20: |
We should work backwards... The nitrile has been made via a nucleophilic substitution of a chloride which in turn was made from an alcohol with thionyl chloride. Since the substitution of the chloride and the reaction of the alcohol are both SN2 reaction there will be two inversions of stereochemistry at that center. Since the product is cis, the starting alcohol needs to be cis. |
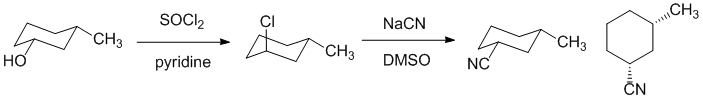 |
| Qu21: |
We should work forwards.... starting from an aldehyde we need to add 2 more C atoms to extend the chain. We can do this with an alkylation of an enolate. This requires that we react the aldehyde with a base to make the enolate and then add the C2 alkyl halide. |
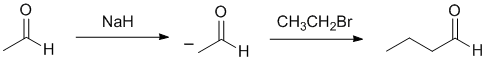 |
| Qu22: |
An alkyl halide reacts with strong base / heat to undergo an E2
elimination of the alkyl halide. For cyclohexane systems, this means that the leaving group and adjacent H need to be axial (in order to be in the preferred 180 degree anti conformation) and this can dictate the regiochemistry (as it does here). |
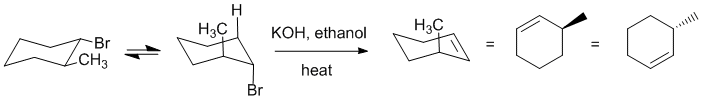 |
| Qu23: |
We should work forwards ... An alcohol to the more highly substituted Zaitsev alkene can achieve by an acid catalysed dehydration of the alcohol via an E1
elimination. |
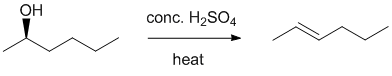
|
| Qu24: |
We should work forwards....Alcohols react with HCl to give alkyl chlorides via an SN1 reaction, i.e. react with an acid (HCl). Here, the alcohol is made into a better leaving group with an acid and the chloride ion reacts as a nucleophile with the C+ to give the racemic alkyl chloride (chloride ion can react with either face of the planar C+). |
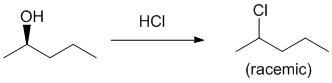 |
| Qu25: |
We need to work backwards.... the product is an ether, which can be made from an alcohol (Nu) and an alkyl halide. Since this is a cyclic ether, we need an intramolecular reaction. Remember to count C atoms. |
 |
| Qu26: |
We should work forwards....Alcohol to alkyl halide where the location of the functional group has changed suggests a carbocation rearrangement has occurred and therefore we require SN1 reactions, i.e. react with an acid (HCl). Here, the alcohol is made into a better leaving group with an acid (promoting SN1 via C+ with hydride shift) and the chloride ion reacts as a nucleophile with the C+ to give the alkyl chloride.
. |
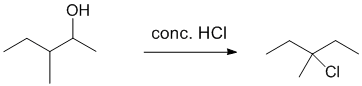 |
CONFORMATIONAL ANALYSIS:
Understanding the terminology and the
energies involved in
conformational
analysis.
Qu 27:
Draw out the most stable conformation of cis-1,3-dimethylcyclohexane... this will have the two methyl groups on the same face of the ring and both equatorial which means there are 6 - 2 = 4 equatorial H.
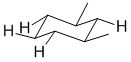
Qu 28:
2-methylbutane is a C5 system, (CH3)2CHCH2CH3. One of the drawn structures is C6, another is C4 (count the C in the alkyl groups and remember +1 for each of the C in the bond we are viewing along).
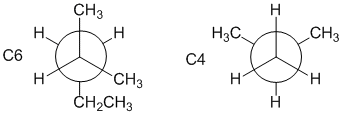
Qu 29:
Torsional strain is caused by eclipsed bonds, therefore torsional strain is reduced in staggered conformations. The boat conformation is eclipsed along the sides of the boat and smaller rings (C5 or less) are not flexible enough to eliminate all the eclipsing interactions and hence all the torsional strain. The staggered conformation of butane has minimal torsional strain.
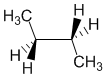
Qu30:
The torsional angle between the chlorine atoms is 120 degrees in this eclipsed conformation.
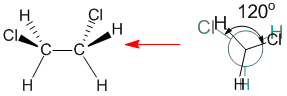
Qu31:
Only 1 of the 5 structures drawn is trans-1,3-dimethylcyclohexane. Trans requires that the two methyl groups are on opposite faces of the ring.
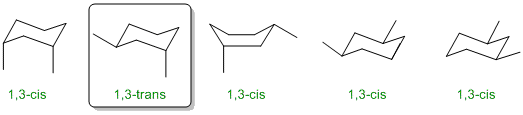
Qu32:
Since we are looking at a cyclopentane where the two methyl groups are on opposite faces of the ring, the best term is trans. Trans requires that the two methyl groups are on opposite faces of the ring.
Qu33:
The structures are isomeric (same MF) but what type of isomer are they? Comparing the drawings or using models and comparing allows one to determine that the -F substituted C both have the same configuration (S) while the -OH substituted C have opposite configurations (Newman R, wedge hash S) and hence they are best described as diastereomers. By redrawing the wedge hash diagram as a Newman projection to compare it with the Newman projection provided, we can see that the front C has the opposite configuration while the back C has the same.
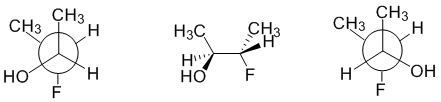
Qu34:
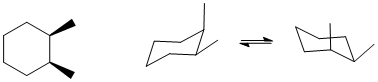
In order for the disubstituted cyclohexane to have two chair conformations with the same energy, the two groups should be the same and there needs to be one axial and one equatorial.
SPECTROSCOPY:
Use any IR information to get the
functional groups. Use the H-NMR
to get the number of types of H, how many of each type from the
integral
and what they are next to from the coupling patterns. Chemical shifts
should
tell you if the group is near -O- or maybe C=O groups etc.
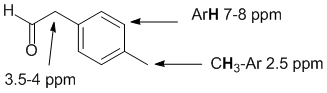
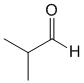
| Qu 35: |
IR shows a carbonyl (i.e. C=O) at 1715 cm-1 which is typical for a ketone as is the C-NMR peak at 204 ppm. The H-NMR has 3 types of H. The 6H doublet at 1.1 ppm,
suggests 2 x -CH3 group, the 1H multiplet at 2.7 ppm a CH and the 1H quartet at 9.7ppm in suggesting an aldehyde. This pieces of data are only consistent with 2-methylpropanal. |
 |
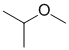
| Qu36: |
IR shows no carbonyl (i.e. C=O) near 1715 cm-1 or in the C-NMR (near 200ppm). IR absorption at 1210 cm-1 could indicate a C-O. There is no OH or NH above 3000 cm-1. The H-NMR has 3 types of H. The 6H doublet at 1.1 ppm,
suggests 2 x -CH3 groups, the 1H septet at 3.5 ppm a CH: these pieces suggest the presence of isopropyl group, -CH(CH3)2. The 3H singlet at 3.2 ppm an deshielded isolated -CH3 group suggesting a methyl ether -OCH3 group. The absence of the carbonyl rules out an ester, so the structure is the ether. |
 |
| Qu37: |
IR shows a carbonyl (i.e. C=O) at 1740 cm-1 and in the C-NMR (at 173 ppm) which suggests a carboxylic acid or derivative. The H-NMR has 3 types of H. The 6H doublet at 1.1 ppm,
suggests 2 x -CH3 groups, the 1H septet at 3.5 ppm a CH: these pieces suggest the presence of isopropyl group, -CH(CH3)2. The 3H singlet at 3.2 ppm an deshielded isolated -CH3 group suggesting a methyl ether -OCH3 group. Due to the presence of the carbonyl group the most likely structure is the methyl ester. |
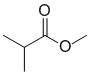 |
| Qu38: |
IR shows no carbonyl (i.e. C=O) near 1715 cm-1 or in the C-NMR (near 200ppm). IR absorption at 1609 and 1502 cm-1 could indicate a aromatic C=C. There is no OH or NH above 3000 cm-1. The C-NMR has 4 types of C, 3 of which (111, 119 and 136 ppm) are in the aromatic region. The H-NMR has 2 types of H. The 6H singlet at 3.1 ppm,
suggests 2 x CH3 groups, and the 2H multiplet at 6.4-6.6 ppm indicate aromatic H. Of the two aromatic systems, the number of types of aromatic C indicates the ortho substitution pattern. |
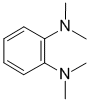 |
| Qu39: |
IR shows no carbonyl (i.e. C=O) near 1715 cm-1 or in the C-NMR (near 200ppm). IR absorption at 3415 cm-1 suggests -OH or -NH. The C-NMR shows only 2 C types, likely sp3. The H-NMR has 3 types of H. The 12H doublet at 1.1 ppm,
suggests 4 x -CH3 group, the broad and exchangeable 1H at 2.0 ppm is consistent with the -OH or -NH. The 2H septet at 3.0 ppm leads to the conclusion that we have 2 x isopropyl group, -CH(CH3)2. Overall, this leads us to the diisopropyl amine. |
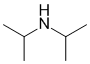 |
| Qu40: |
IR shows a carbonyl (i.e. C=O) at 1735 cm-1 and in the C-NMR (at 170 ppm) which suggests a carboxylic acid or derivative. The H-NMR has 3 types of H. The 6H doublet at 1.3 ppm,
suggests 2 x -CH3 groups, the 3H singlet at 2.2 ppm an slightly deshielded isolated -CH3 group and the 1H septet at 4.9 ppm a CH: these pieces suggest the presence of isopropyl group, -CH(CH3)2 that is attached to an electronegative atom (O ?). Due to the presence of the carbonyl group the most likely structure is the isopropyl ester. |
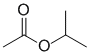 |