Part 7: MECHANISMS
Note that no other reagents are needed in order to complete any
of these sequences, you should only be using what is there.
Common errors:
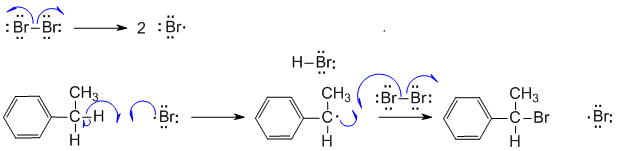
A. Not showing a radical reaction, not showing the correct sequence for the radical reaction.
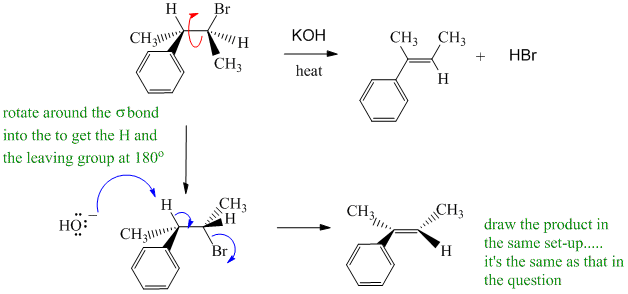
B. Showing the reaction as SN instead of E (which are favoured with heat), E1 instead of E2 (strong base promotes E2), ignoring the stereochemistry of the E2 process.
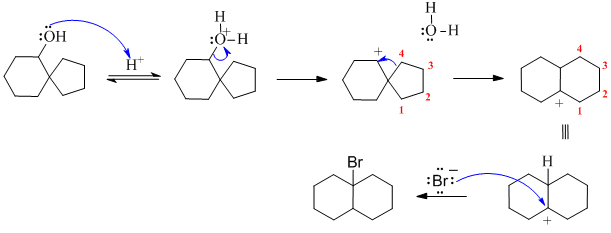
C. Not showing the reaction as SN1, not being able to draw the arrows for the 1,2-alkyl shift.
Links to other examples of the same reactions are provided.
A
Radical bromination at the benzylic position (which gives the most stable radical, secondary with resonance).

B
E2 reaction of an alkyl bromide using a strong but small base (HO-) implies it should follow Zaitsev's rule by removing the H at the tertiary C (benzylic). Need to address the stereochemistry by redrawing with the H and the Br anti (i.e. 180 degrees) to show that this results in the alkene with the methyl groups cis to each other.
C
Looking at the reaction, -OH becomes -Br, but location has changed and so has the hydrocarbon skeleton....this tells us carbocation rearrangement has occurred during a nulceophilic substitution so we have SN1. So, protonate the OH with H+ to make a better leaving group, then make it leave to give the secondary C+. A 1,2-alkyl shift will then give a more stable tertiary C+ and the right bicyclo fused hydrocarbon skeleton. Attack of the Br- as the nucleophile on the C+ gives the product.