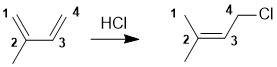
Here is an post-mortem analysis / "how to" for the Final. The questions are split by the sections. At the start of each section are a few suggestions of what to look for or how to tackle the question type.
RELATIVE PROPERTIES:
Identify the controlling feature, which is not
always as obvious as it may appear. Look for two pairs of similar systems to compare
that have minimal differences in structure. If a compound is named, draw it out.
If a reaction is involved, identify the type of reaction and then what the controlling
factors are.
Qu1:
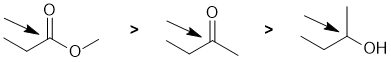
Oxidation states...The easiest way to reach a partial answer is based on the knowledge that ketones can be reduced to secondary alcohols. More bonds to O (or more electronegative atoms) typically means a higher oxidation state. More formally, we count the bonds attached to the atom being considered. A bond to a more electronegative atoms (e.g. C attached to O or N) counts -1, a bond to the same type of atom counts 0 and a bond to a less electronegative atom (e.g. H) counts +1. Total the count and then consider the formal charge on the central atom since the oxidation state for the central atom plus the groups attached must equal the atoms formal charge.
Ketone: the C is attached to 2 x O (count - 1 each, total -2) and 2 x C (count 0) therefore total = -2 and therefore the oxidation state C = +2.
Alcohol: the C is attached to 1 x O (count - 1 each, total -1) and 2 x C (count 0) and 1 x H (count +1) therefore total = 0 and therefore the oxidation state C = 0.
Ester: the C is attached to 3 x O (count - 1 each, total -3) and 1 x C (count 0) therefore total = -3 and therefore the oxidation state C = +3.

Qu2:
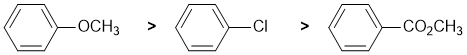
The reaction is electrophilic aromatic substitution, nitration and we need to look at the substituent effects on the aromatic ring. The substituents are a methoxy group, an ester connected via the carbonyl C=O and chloro group. The ester attached through the carbonyl group is a moderate electron withdrawing group, a moderate deactivator due to resonance effects. The methoxy group is a strong electron donor which is strongly activating due to resonance effects. Chloro groups is weakly electron withdrawing (and deactivating) due to the inductive effects of the electronegative halogen atoms. So the reactivity:
Qu3:
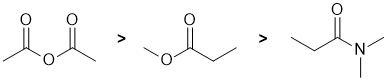
The reactivity of carbonyl groups in carboxylic acid derivatives towards aq. acid where the systems undergo nucleophilic acyl substitution. The electronic effect and leaving group ability of each of the substituents on each of the carbonyl groups need to be considered. Electron withdrawing groups on the carbonyl make the carbon more electrophilic (and hence more reactive). Better leaving groups also increase the reactivity of the acid derivative. Hence, in terms of reactivity towards aq. acid, acid anhydrides are more reactive than esters which in turn are more reactive than amides, i.e. we have:
Qu4:
Acidity...Remember the lower the pKa the stronger the acid, and think of the simple acid equation HA <=> H+ A- then look for factors that stabilise A-.... The simplest system is the cyclic ester that when deprotonate gives a simple ester enolate (pKa = 25) due to the resonance stabilisation of the conjugate base by the carbonyl group. The two cyclic polyenes look similar. Cyclopentadiene (pKa = 16) when deprotonated gives a conjugate base that is aromatic (a 6 pi system, hence stabilised). Cycloheptatriene (pKa = 36) when deprotonated gives a conjugate base that is not aromatic (lacks stabilisation).
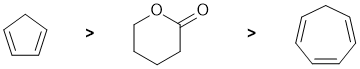
Qu5:
The reaction is the Diels-Alder reaction and we are looking at the dieneophiles reacting with the diene (1,3-butadiene). The reactivity increases in the Diels-Alder reaction with electron withdrawing groups on the dienophile. Here we have an alkyl group (weak electron donor), an nitrile (strong electron withdrawer) and an alkoxy group (strong electron donor). Therefore :
![]()
Qu6:
Basicity...Remember the lower the pKa the stronger the acid (hence weaker base), and think of the simple acid equation HA <=> H+ A- (where A- is the base we are thinking about) look for factors that affect the electron availability of A-.... Here we are looking carbonyl systems. one simple carbonyl and two dicarbonyls (active methylene compounds have more resonance stabilisation, the electrons are less available, they are less basic). In the simple ketone, the removal of H from the C atom next to the C=O allows for resonance stabilisation of the -ve charge to the electronegative O atom (pKa is about 20). In the dicarbonyls, we have a 1,3-diester and a 1,3-ketoester. First let's compare a simple ester to the simple ketone. An ester is similar to a ketone, but there is competing resonance from the alkoxy O atom so there is less ability to stabilise the -ve charge, so esters are less acidic than ketones (ester pKa about 25). Hence in the 1,3-dicarbonyl systems, the diester is more basic than the keto-ester (pKas about 13 and 11 respectively). Therefore, in terms of basicity:
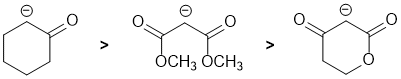
Qu7:
This question relates to resonance energy and aromaticity. Aromatic systems have high resonance stablisation and therefore high resonance energies. Conjugated systems also have resonance stabilisation but not to the same extent as aromatics. These 3 systems were discussed when we discussed how to evaluate resonance energy and the aromaticity of benzene (see the figure in the link above). Here we have three dienes, one isolated and two conjugated, but one is also a heteroaromatic. Hence, it terms of resonance energies:
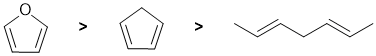
Qu8:
Ring opening of epoxides with strong nucleophiles occurs in an SN2 fashion with the Nu- attacking at the least hindered end... This gives the major product. If the Nu- attacks the less hindered end we get a minor product and the third can't be formed under these conditions.
AROMATICITY and RESONANCE:
Best method is to work through the molecules and decide on the aromaticity based on the four criteria for the pi system (cyclic, planar, conjugated pi system with 4n+2 pi electrons).
Qu9:
Decide on the aromaticity of each molecule based on the four criteria for the pi system (cyclic, planar, conjugated pi system with 4n+2 pi electrons). In terms of the Huckel rule ( 4n + 2 pi electrons), if n is not 1then we likely need either 2 (n=0) or 10 (n=2) pi electrons (given the choices available), but the only option is the 10 pi (n = 2) molecule :

Qu10:
To be non-aromatic as drawn but with an aromatic resonance structure, we need to find a system that satisfies all the criteria for aromaticity except the electron count and we can therefore add / subtract electrons via resonance. Perhaps most critically, it means that all the atoms in the cyclic structure must already contribute to the p system. So in these examples, it means they are all sp2 hybridised.
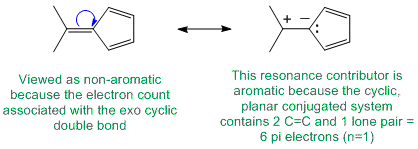
Qu11:
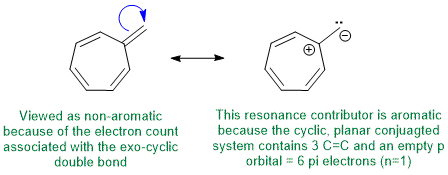
To be non-aromatic as drawn, we need to find a system that fails on one of the first three criteria (i.e. it lacks a cyclic conjugated pi system) but when protonated (i.e. has a conjugate acid) that satisfies all four of the criteria for aromaticity (i.e. it has a pi system that is cyclic, planar, fully conjugated and contains 4n+2 pi electrons). The most likely scenario will involve addition of H+ to form an aromatic carbocation:
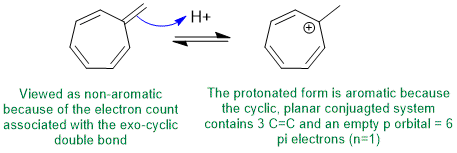
Qu12:
To be non-aromatic as drawn, we need to find a system that fails on one of the first three criteria (i.e. it lacks a cyclic conjugated pi system) but has a conjugate base that satisfies all four of the criteria for aromaticity (i.e. it has a pi system that is cyclic, planar, fully conjugated and contains 4n+2 pi electrons). The most likely scenario will involve loss of H+ from a sp3 center to create a conjugated lone pair (so adding 2 electrons to the pi system) ... for clarity the aromatic resonance structures of the conjugate bases are also shown:
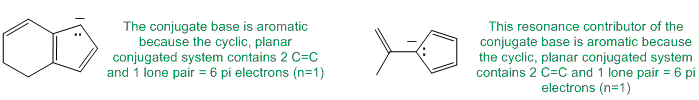
Qu13:
The most common form of tautomerism switches H and double bond positions... To be non-aromatic as drawn, we need to find a system that fails on one of the first three criteria (i.e. it lacks a cyclic conjugated pi system) but has a tautomer that satisfies all four of the criteria for aromaticity (i.e. it has a pi system that is cyclic, planar, fully conjugated and contains 4n+2 pi electrons):
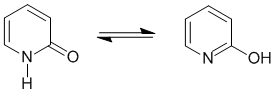
Qu14:
Compounds that are aromatic when deprotonated have aromatic conjugate bases that satisfies all four of the criteria for aromaticity (i.e. it has a pi system that is cyclic, planar, fully conjugated and contains 4n+2 pi electrons). The most likely scenario for these examples will be loss of H+ from NH systems... for clarity the aromatic resonance structures of the conjugate bases are also shown:
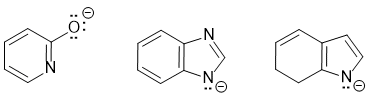
Qu15:
In order to be anti-aromatic, we need a system that meets the first 3 criteria but has 4n pi electrons (an even number of pi electron pairs). In this case assuming the molecule is planar (as stated in the question), there then would be 3 C=C plus N lone pair = 8 pi electrons (even number of pi electron pairs):
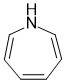
Qu16:
Decide on the aromaticity of each molecule based on the four criteria for the pi system (cyclic, planar, conjugated pi system with 4n+2 pi electrons). In terms of the Huckel rule ( 4n + 2 pi electrons), if n is not 1then we likely need either 2 (n=0) or 10 (n=2) pi electrons (given the choices available), but the only option is the 10 pi (n = 2) molecule :
STARTING MATERIALS AND PRODUCTS:
If you are trying to find the product, then you should probably just work forwards through the sequence of reactions.
If you are looking for the starting material, then working backwards is probably the best way to go....
Basically depends on the need to know and identify the reactions, this is often triggered by looking at the functional groups in the molecules.
Qu17:
Working forwards, the first step is an electrophilic aromatic substitution, specifically Friedel-Crafts alkylation that will be para on the more electron rich (activated ring). This is followed by aromatic nitration ortho to the stronger donating group and hence meta to the alkyl group on the more activated ring.
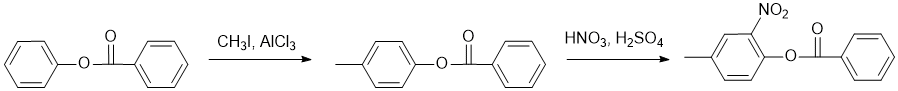
Qu18:
Working backwards, we have a product that is a highly substituted cyclohexane with specific stereochemistry : both are indicators of a Diels-Alder reaction. This would be consistent with the starting material shown, a good dienophile and step 1 just being "heat". We need to identify the conjugated diene. Step 2 reduces the cyclohexene to the cyclohexane. Make sure to count C atoms to get the right substitution on the diene and also the right stereochemistry.
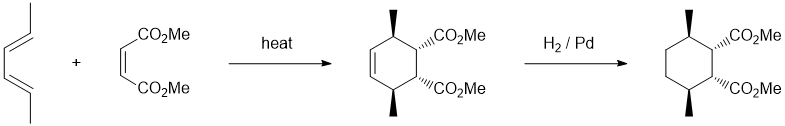
Qu19:
Working backwards, we have a benzoic acid that was likely formed by oxidation of a benzylic position (so an alkyl group with benzylic H) that was created via a Friedel-Crafts alkylation:
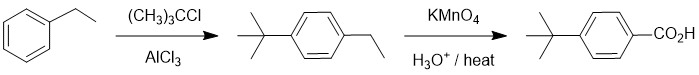
Qu20:
Working forwards, the first step is the reduction of the carbonyl groups (an aldehyde and an ester) to give 1,3-propanediol then reaction of the diol to give a cyclic ketal :
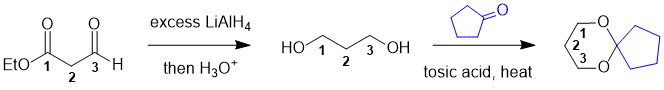
Qu21:
Working forwards, the first step is an electrophilic aromatic substitution, specifically an alkylation via a carbocation intermediate (formed via alkene protonation). This is followed by aromatic bromination para to the alkyl group.
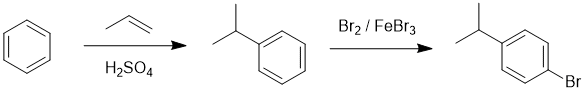
Qu22:
Working backwards, we have a product that is a ketone formed after the normal work up of a Grignard reaction. Carbonyl systems (except for carboxylic acids) tend to give alcohols (remember that ketones are more reactive than esters so one can't stop it in the middle) while carboxylic acids undergo simple acid / base reactions. On the other hand, nitriles form ketones...
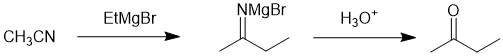
Qu23:
Working backwards.... The product is a cyclic system and a ketone. Looking at the first reaction we have a 1,3-dicarbonyl system being treated with a base which suggests the formation of an enolate from the active methylene. The ring has been formed by the double alkylation (count C atoms, think about curly arrows and connectivity). Step 2 (aq. acid / heat) has caused (a) ester hydrolysis then (b) decarboxylation of the beta-ketoacid.
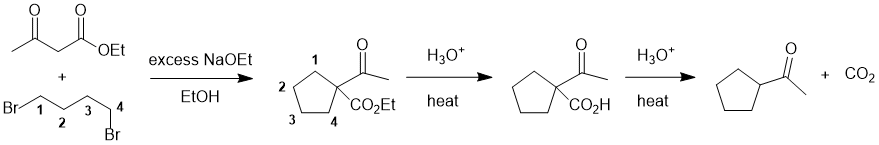
Qu24:
Working forwards, the reaction is the formation of an epoxide from a halohydrin via an intramolecular SN2 (where the Nu (OH) needs to attack at 180 degrees to the Cl (LG)). It's best to draw (or better still build a model) of the starting material in the reactive conformation to keep careful track of the stereochemistry:
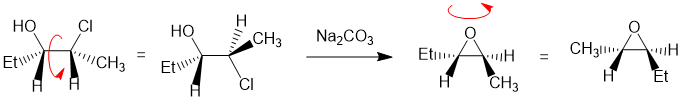
REAGENTS FOR SYNTHESIS:
Need to be able to look at reactions, looking at the functional groups in the starting materials and products of each step to think about
how you have got there.
How well do you know your reagents
? Look at what has actually happened in terms of the reaction functional
group transformation and then first look for any regiochemical issues
then finally the stereochemistry last (it's the hardest to sort out).
Qu25:
Need to deal with the stereochemistry... which requires the cis-alkene to be used to create the right epoxide stereochemistry then adding the methoxy group at the less hindered end by using SN2 like (basic) conditions and a strong nucleophile. It's best tackled with wedge-hash drawings of the product or better still using a model kit.
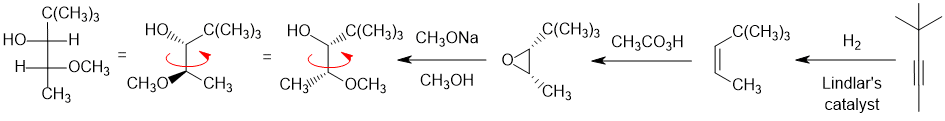
Qu26:
Introduce the phenolic -OH group via diazonium chemistry (that starts from the nitro group : reduce, neutralise and then diazotise...).
Qu27:
The terminal alkyne can be converted into an aldehyde (via hydration) then the aldehyde reacted with a Grignard reagent to yield the secondary alcohol (count C atoms):
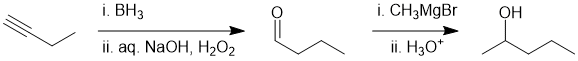
Qu28:
The amide is converted to the amine by hydrolysis but best to brominate first to avoid poly-bromination (since the amide is a weak activator than a simple amine):
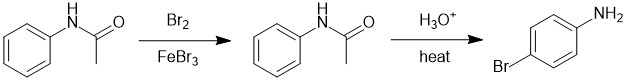
Qu29:
Count C atoms => C4 to C4 so no new C atoms... hence alkene to primary alcohol ( hydroboration-oxidation of the alkene), then oxidation of the primary alcohol to the carboxylic acid:
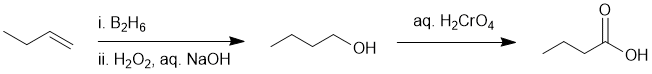
EXPLANATION OF PHENOMENA
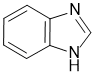
Qu30:
All about the nature of the N atoms in the heteroaromatic compound imidazole. The N lone pair that is not part of the aromatic system is the one that is more basic.
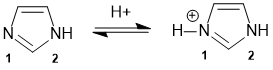
Qu31:
The oxidation of alcohols as chromate esters occurs during an elimination that requires to removal of an H atom beta to the chromate ester leaving group.
Qu32:
Internal alkynes react with excess HCl to give geminal dihalides. The first equivalent of HCl reacts to give a vinyl chloride. When the 2nd equivalent of HCl reacts, it protonates to give the more stable carbocation intermediate.
Qu33:
In the more acidic amide, there is at least one H attached to the N atom (remember that N is electronegative and can stabilise the conjugate base). In the other amide, the N has only C atoms attached and the most acidic H is alpha to the carbonyl group.
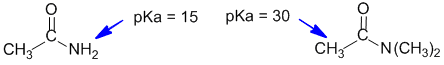
Qu34:
All about kinetic and thermodynamic control in the addition of HCl to conjugated dienes. Here we see 1,4-addition of the HCl, which had provided the more highly substituted alkene which is the more stable alkene and hence the thermodynamic product which tends to be favoured at higher temperatures.