Here is an post-mortem analysis / "how to" for the Final. The questions are split by the sections. At the start of each section are a few suggestions of what to look for or how to tackle the question type.
RELATIVE PROPERTIES:
Identify the controlling feature, which is not
always as obvious as it may appear. Look for two pairs of similar systems to compare
that have minimal differences in structure. If a compound is named, draw it out.
If a reaction is involved, identify the type of reaction and then what the controlling
factors are.
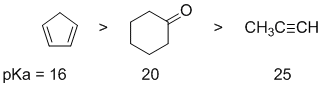
Qu1:
Acidity...Remember the lower the pKa the stronger the acid, and think of the simple acid equation HA <=> H+ A- then look for factors that stabilise A-.....In all three cases we are looking at C-H systems. We have a terminal alkyne, a ketone and a cyclic diene. For the terminal alkyne, the C-H bond is an sp C-H bond. The carbanion is stabilised because the -ve charge is associated with an sp hybrid orbital and is close to the +ve nucleus. In the ketone, deprotonation of an sp3 C-H in the alpha position (next to the carbonyl group) gives a resonance stabilised anion. For cyclopentadiene, the conjugate base is aromatic and the resonance stabilisation by the aromatic system in is the most significant, followed by the sp effect in the terminal alkyne (if you know your pKa's then this is easy : terminal alkyne about 25, ketone about 20 and cyclopentadiene about 16. So for strongest acid to weakest acid:

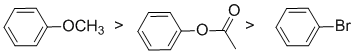
Qu2:
The reaction is electrophilic aromatic substitution, Friedel-Crafts alkylation, and we need to look at the substituent effects on the aromatic ring. The substituents are a methoxy group, an ester and a bromine. The alkoxy group is a strong electron donating group via resonance donation of the O lone pair - it's a strongly activating group. Note that the ester group is attached through the alkoxy O and therefore is a moderate electron donating group via resonance donation of the O lone pair - it's a moderately activating group. The halogen group (bromine) is a slightly deactivating group due to inductive effects (due to electronegativity). So the reactivity:
Qu3:
The reactivity of CC pi bonds towards catalytic hydrogenation. The three functional groups arene, alkene and alkyne. The reactivity towards reduction is determined by the strength of the pi bonds: the weaker the pi bond, the faster the reaction. So alkynes reduce more rapidly than alkenes and aromatic C=C reduce more slowly than alkene C=C due to the aromatic stabilisation. So therefore the reactivity order:
![]()
Qu4:
Two of the three systems are substituted alkenes so we are looking at electrophilic addition to an alkene. The third system is an alkane... quite unreactive to acids are there are no available electrons in lone pairs or pi bonds.... we only talked about radical substitution of alkanes. A closer look at the two alkene structures with different substituents, an alkoxy group -OR, and an alkyl group -R. Each alkene will react with the acid to give a carbocation and the substituent will affect the availability of the pi electrons: electron donating substituents will make them more available and electron withdrawing will make them less available. Or we can look at carbocation stability.... an electron donor will stabilise a carbocation, an electron withdrawer will destabilise a carbocation. Looking at the substituents: an alkoxy group, -OR, is a strong electron donor, and an alkyl group, -R, is a weak electron donor.
![]()
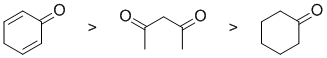
Qu5:
The % of the enol present in solution is influenced by the stability of that enol. For a simple ketone, such as cyclohexanone, the enol form is not particularly stable and the ketone (carbonyl) form dominates (only about 2% enol). In a 1,3-dicarbonyl, intramolecular H-bonding in the enol form helps stablise the enol (about 20%). Cyclic dienones tautomerise to give an aromatic alcohol, phenol, where the enol has aromatic stability (100% enol).
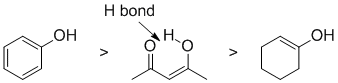
Qu6:
Resonance energy indicates the stability of the conjugated system compare to a non-conjugated system. Aromatic systems have high resonance stablisation and therefore high resonance energies. Conjugated systems also have resonance stabilisation but not to the same extent as aromatics. Benzene, perhaps our "model" aromatic compound, has 3 C=C with resonance energy = 36 kcal/mol. Naphthalene has a 5 C=C in conjugation and therefore it will have more resonance energy (61 kcal/mol) than benzene itself. Cyclohexa-1,3-diene which is not aromatic, is just a conjugated diene (4 kcal/mol) . Hence:
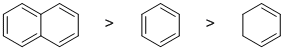
Qu7:
Oxidation states...The easiest way to reach the answer is based on the knowledge that ketones can be reduced to alcohols. More formally, we count the bonds attached to the atom being considered. A bond to a more electronegative atoms counts -1, a bond to the same type of atom counts 0 and a bond to a less electronegative atom (e.g. H) counts +1. Total the count and then consider the formal charge on the central atom since the oxidation state for the central atom plus the groups attached must equal the atoms formal charge. For the ketone, the C is attached to 2 x O (count - 1 each, total -2) and C (count 0) therefore total = -2 and therefore the oxidation state C = +2. In the alcohol, the C is attached to 1 x O (count - 1), 2 x C (count 0) and H (count +1) therefore total = 0 and therefore the oxidation state C = 0. In the alkane the C is attached to 2 x C (count 0), C and 2xH (count +2) therefore total = +2 and therefore the oxidation state C = -2.
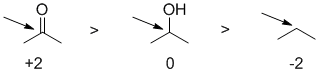
Qu8:
The reaction is an example of electrophilic aromatic substitution, a bromination. We need to look at the substituent effects on the aromatic ring. An alkyl group-butyl group (-R) is slightly electron donating groups via hyperconjugation - it's an activating group and ortho/para directing. Due to its size, sterics will favour para over ortho since the ortho sites are partially blocked.
para > ortho > meta
Qu9:
The reactivity of carboxylic acid derivative carbonyl groups of an acid chloride, an ester and an amide towards hydrolysis. The electronic factors of each of the substituents / leaving group on each of the carbonyl groups need to be considered. In the chloride, is an electron withdrawer (inductive) and a good leaving group. In the ester, the -OR group, the methoxy group is a strong electron donor and a poor leaving group. In the amide, the NHR group, is a strong electron donating group and a very poor leaving group. Electron donors on the carbonyl make the carbon less electrophilic and less reactive, a better leaving group makes the acid derivative more reactive. Hence in terms of reactivity:
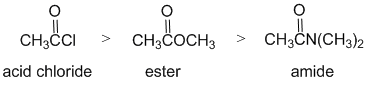
Qu10:
Configurational isomers result at chirality centers (R or S) and double bonds (E or Z) (note this question was also on your MT....)
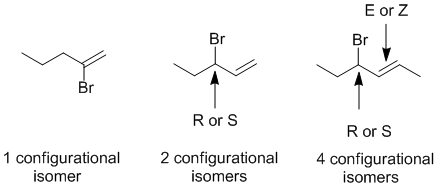
STRUCTURES and PROPERTIES:
You need to know about functional groups and reactions, and how to apply concepts related to structure such as stereochemistry, acidity/basicity and reactivity etc.
Qu11:
Phenol (pKa = 10) is more acidic than a simple alcohol (pKa =15ish) or the carbonyl systems (pKa 17 (aldehydes), 20 (ketones) 25 (esters)).
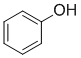
Qu12:
A precipitate from reaction with 2,4-dinitrophenylhydrazine indicates the formation of the hydrazone from the reaction with either an aldehyde or a ketone. A precipitate from the iodoform test indicates the presence of a methyl ketone.
![]()
Qu13:
A precipitate from reaction with 2,4-dinitrophenylhydrazine indicates the formation of the hydrazone from the reaction with either an aldehyde or a ketone. A precipitate (silver mirror) from the Tollen's test indicates the presence of an aldehyde.
![]()
Qu14:
An orange solution from reaction with 2,4-dinitrophenylhydrazine indicates neither an aldehyde or a ketone. If it has a carbonyl group, C=O, then it is the ester.
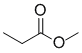
Qu15:
A precipitate with the ferric chloride test indicates the presence of a phenol.

Qu16:
We need to look at the substituent effects on the aromatic ring. Groups attached via a heteroatom with a lone pair can be resonance donors (activated), and the less electronegative that atom is, the better it is as a donor, hence the amine (aniline) is the most activated system.
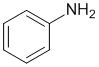
Qu17:
We need to look at the substituent effects on the aromatic ring. Groups attached via an atom that is partially or formally +ve and / or attached to more electronegative atoms, will be electron withdrawing (deactivating). Since the N in a nitro group is both formally +ve and has 3 bonds to a more electronegative oxygen atom, it is a very strong electron withdrawer.
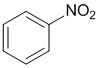
Qu18:
We need to look at the substituent effects on the aromatic ring. Groups attached via an atom that is partially or formally +ve and / or attached to more electronegative atoms will be electron withdrawing (deactivating) and direct meta.... the exceptions are halogens (deactivating due to their electronegativity) which direct ortho / para.
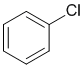
Qu19:
Friedel-Crafts acylation only work on systems that at are at least as reactive as halobenzenes, i.e. they fail for the more deactivated systems. But they also fail for amines because the amino group reacts with the Lewis acid catalyst to become a strong deactivator.
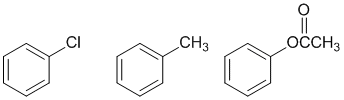
AROMATICITY and RESONANCE:
Best method is to work through the molecules and decide on the aromaticity based on the four criteria for the pi system (cyclic, planar, conjugated pi system with 4n+2 pi electrons).
Qu20:
The least stable type of diene (two alkenes) is an isolated diene.
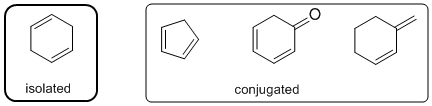
Qu21:
To be non-aromatic as drawn, we need to find a system that fails on one of the first three criteria (i.e. it lacks a cyclic conjugated pi system). The most common form of tautomerism switches H and double bond positions and the involvement of carbonyl groups is quite common, tautomers of ketones are the enols... which means we could move a pi bond into the cyclic structure.
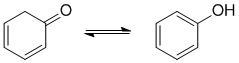
Qu22:
Monocyclic means 1 ring...To be non-aromatic as drawn but with an aromatic conjugate base, we need to find a system that becomes aromatic when we remove a proton and form the conjugate base typically adding another pair of electrons to the pi electron count for the Huckel rule.
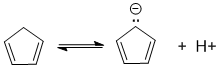
Qu23:
Bicyclic means 2 rings. If the conjugate base gains aromaticity, it will make the hydrogen more acidic (e.g. cyclopentadiene systems) but the acidity will be further enhanced if there is additional resonance delocalisation.
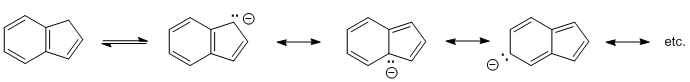
Qu24:
Bicyclic means 2 rings. Need to identify a conjugated diene that can react in a Diels-Alder reaction to give an aromatic product.
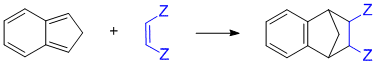
Qu25:
To be non-aromatic as drawn, we need to find a system that fails one or more of the first 3 criteria for aromaticity (i.e. it lacks a cyclic conjugated pi system). There are two non-aromatics (pick one) in the set (both contain sp3C in the rings), the rest are all aromatic.
![]()
Qu26:
The compound with the greatest resonance energy is should be aromatic and have the longest conjugated system.
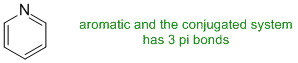
Qu27:
Look for an aromatic with the most heteroatoms where the lone pairs are not involved in the pi system.
![]()
Qu28:
To be aromatic as drawn but with a non-aromatic conjugate acid, we need to find a system that is aromatic as drawn yet when it reacts with H+ is no longer aromatic. A typical example would be where a lone pair of a heteratom is initially part of the aromatic pi system but those electrons are used to make the new bond to the H+.
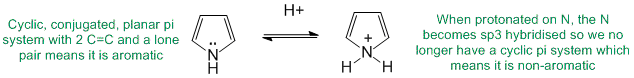
Qu29:
In order to be sp3 hybridised, the heteroatom needs to be only involved in single bonds and not be interacting through resonance with an adjacent pi system (i.e. not conjugated).
![]()
STARTING MATERIALS AND PRODUCTS:
If you are trying to find the product, then you should probably just work forwards through the sequence of reactions.
If you are looking for the starting material, then working backwards is probably the best way to go....
Basically depends on the need to know and identify the reactions, this is often triggered by looking at the functional groups in the molecules.
Qu30:
Working forwards, treatment of a 1,2-halohydrin with sodium carbonate as a base forms the epoxide via an SN2 reaction. First convert the Fischer projection into a wedge hash diagram and then set up the starting material in the conformation in which is reacts where the HO and the Br are anti (180 degrees) to each other so that the Nu can attack the backside (i.e. at 180 degrees to the LG) and cause the normal SN2 inversion resulting in the methyl and ester groups being trans- to each other.
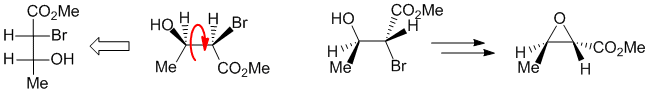
Qu31:
Working forwards, the terminal alkyne undergoes hydroboration / oxidation to give the aldehyde. Addition of a methyl group to the aldehyde carbonyl with an organometallic reagent gives a secondary alcohol. This then undergoes PDC oxidation of the secondary alcohol to the ketone and converted to an alkene with a Wittig reaction.
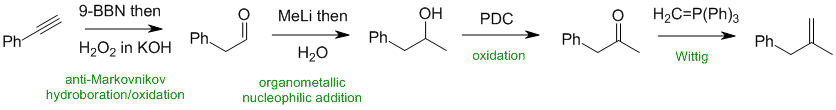
Qu32:
Working forwards, the first step is an electrophilic
aromatic substitution, specifically nitration followed by chlorination to give the meta product. Reduction of the nitro group converts it to the amine which is then converted in to the amide.
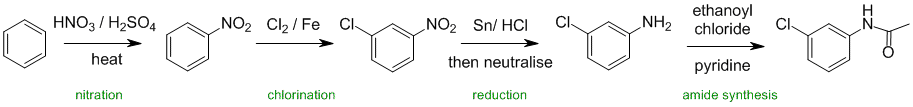
Qu33:
Working forwards, the first step is the synthesis of a carboxylic acid using a Grignard reagent. This is followed by Fisher esterification and then partial reduction of the ester to the aldehyde uisng DIBAL.
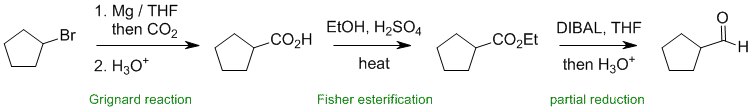
Qu34:
Working backwards.... the peracid looks to have produced the carboxylic acid from a Baeyer-Villager oxidation of an aldehyde, which reveals the enal type structure from an aldol condensation.
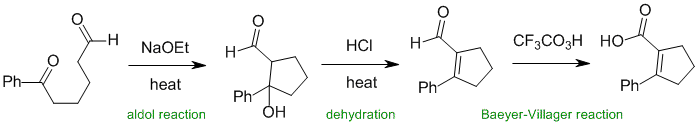
Qu35:
Working forwards, the first step is a Diels-Alder reaction (recognise the conjugated diene and the simple reaction conditions). This is followed by dihydroxylation of the alkenes and then conversion of the 1,2-diol reacts with a ketone to give a cyclic ketal.
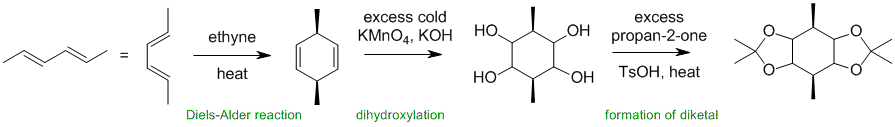
Qu36:
Working backwards.... the last step epitomises the complexity of a simple reagent, a simple acid. Here is promotes several things... alcohol dehydration, ester hydrolysis and decarboxylation. In comparison, step 1 is simpler once you recognise the active methylene compound is forming an enolate and reacting with a carbonyl.
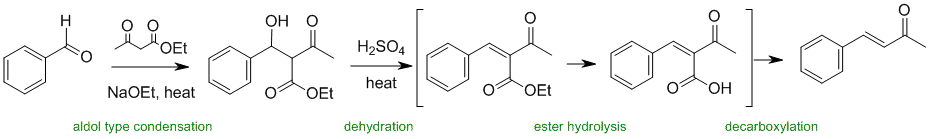
Qu37:
Working forwards....
The first step is an electrophilic
aromatic substitution, specifically Friedel-Crafts
acylation. on the more activated ring (not the nitro substituted ring). This is followed by a Clemmensen reduction to convert the carbonyl group into a methylene. The third step is a another aromatic substitution, bromination (again on the more activated ring) then reduction of the nitro group to the amine.

Qu38:
Working forwards.... reaction of an organometallic reagent with an epoxide (reacts at the least hindered end) which after work up gives a secondary alcohol... count carbons. PDC oxidation of the secondary alcohol to the ketone. The ketone will then undergo a Baeyer-Villager reaction to give the ester.
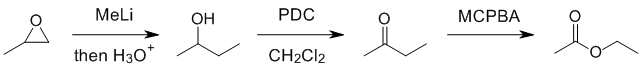
REAGENTS FOR SYNTHESIS:
Need to be able to look at reactions, looking at the functional groups in the starting materials and products of each step to think about
how you have got there.
How well do you know your reagents
? Look at what has actually happened in terms of the reaction functional
group transformation and then first look for any regiochemical issues
then finally the stereochemistry last (it's the hardest to sort out).
Qu39:
Friedel-Crafts
acylation between an acid chloride and benzene to make the aromatic ketone which can then be oxidised to the give the aromatic carboxylic acid. Grignard reagents, RMgX, don't react with benzene rings, and to make a carboxylic acid using Grignard chemistry, you need a halide. We need to use i. CH3Cl / AlCl3 ii. KMnO4 / H3O+ / heat.
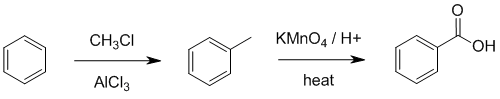
Qu40:
First you need to analyse the stereochemistry of the Fischer projection and use that to work out if you need to do a syn or anti addition to a cis or trans C=C. That analysis shows that we need to do either an anti addition to a cis-alkene or a syn addition to a trans-alkene. Na/NH3 gives trans-alkenes, H2 / Pd will reduce to an alkane and H2 / Lindlar's catalyst gives cis-alkenes. KMnO4 / aq. NaOH results in a 1,2-diol via a syn addition and peracid / H3O+ a 1,2-diol via overall anti addition. Therefore, use i. H2 / Lindlar's catalyst ii. CH3CO3H iii. H3O+
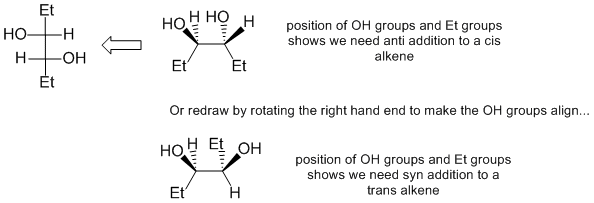
Qu41:
Need to alkylate the terminal alkyne adding just 1 carbon and then reduce to the trans alkene so use i NaNH2, ii. CH3Br, iii. Na / NH3
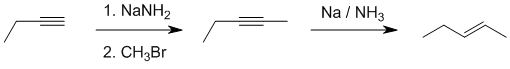
Qu42:
The alkene can be rearranged to a more stable position (more highly substituted) using simple H+. The HBr additions followed by E2 elimination both lead to the original alkene. The Br2 additions followed by E2 elimination both lead to dienes.
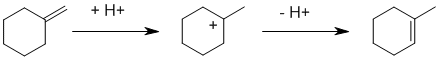
Qu43:
Two reductions are required. The Clemmenson (Zn/Hg and HCl) reduces the aromatic ketone C=O to a CH2 and then LiAlH4 will reduce the amide to the amine.
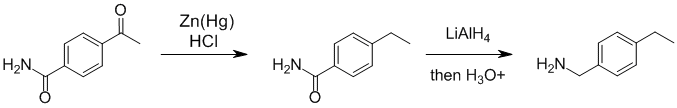
Qu44:
First you need to analyse the stereochemistry of the Newman projection and use that to work out if you need to do a syn or anti addition to a cis or trans C=C. That analysis shows that we need to do either an anti addition to a cis-alkene or a syn addition to a trans-alkene. Na/NH3 gives trans-alkenes, H2 / Pd will reduce to an alkane and H2 / Lindlar's catalyst gives cis-alkenes. KMnO4 / aq. NaOH results in a 1,2-diol via a syn addition and peracid / H3O+ a 1,2-diol via overall anti addition. Therefore, use i. H2 / Lindlar's catalyst ii. CH3CO3H iii. H3O+

EXPLANATION OF PHENOMENA
Qu45:
The dipoles are created by charge distribution. The N on both systems are sp2 hybridised but the orbital location of the lone pairs differs. The resonance contributors for pyrrole require delocalisation of the N lone pair resulting in the +ve charge on the N while the electronegativity of N in pyridine makes the N slightly -ve.
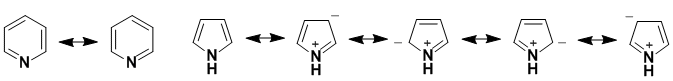
Qu46:
The difference is due to the anti-aromatic character of the cyclic conjugated pi system in structure 1 with the C=C and N lone pair (4 pi electrons, an even number of pairs).

Qu47:
A standard approach to looking at acidity is to look for factors that stabilise the conjugate base (i.e. think about A- in the acid reaction HA <=> H+ and A-). The cis structure of the maleic acid and hence the proximity of the acid groups, means that a favourable intramolecular hydrogen bond can stabilise the conjugate base of maleic acid compared to fumaric acid.
Qu48:
Draw the two structures and compare them.... look for similarities and differences.... both are conjugated dienes, the location of the Br in terms of the nature of the C they are attached to are different but does that explain the difference ? Only in the sense that one of them can eliminate to give a stable aromatic system which would make the reaction favourable...
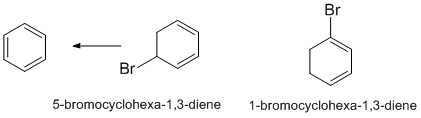
Qu49:
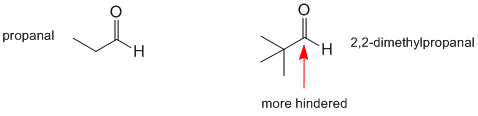
Both are aldehydes, the difference is the nature of the alkyl groups. Aldehyde and ketone reactivity is affected by the electronic effects and the steric effects of the alkyl groups. More branched alkyl groups will hinder the approach of the nucleophiles.