Here is an post-mortem analysis / "how to" for the Final. The questions are split by the sections. At the start of each section are a few suggestions of what to look for or how to tackle the question type.
RELATIVE PROPERTIES:
Identify the controlling feature, which is not
always as obvious as it may appear. Look for two pairs of similar systems to compare
that have minimal differences in structure. If a compound is named, draw it out.
If a reaction is involved, identify the type of reaction and then what the controlling
factors are.
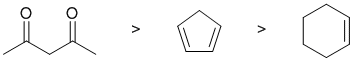
Qu1:
Acidity...Remember the lower the pKa the stronger the acid, or think of the simple acid equation HA <=> H+ A- then look for factors that stabilise A-.....Here we have three C-H systems. We have a cycloalkene, a cyclic diene and a 1,3-diketone. In the diketone, the H on the CH2 in between the two C=O are quite acidic (pKa = 9) due to the two resonance contributors that allow the -ve charge of the conjugate base to be delocalised to each of the electronegative oxygen atoms. For cyclopentadiene, the conjugate base is aromatic and the resonance stabilisation by the aromatic system is significant, pKa = 16. For the cyclohexene, the allylic position will be the most acidic (about 45) where the conjugate base will be a resonance stabilised carbanion. If you know your pKa's then this is easy : 1,3-diketone about 9, cycloalkene about 45 and cyclopentadiene about 16. So for strongest acid to weakest acid:

Qu2:
The reaction is electrophilic aromatic substitution, bromination and we need to look at the substituent effects on the aromatic ring. The substituents are a nitro group, an ester connected via the -O- and a ketone. The nitro group (-NO2) is a strong electron withdrawing group via resonance and induction of the +ve N atom - it's a strongly deactivating group. The ester is attached through the alkoxy O and therefore is a moderate electron donating group via resonance donation of the O lone pair (note that there are competing effects for the interaction of the lone pair towards the arene and the carbonyl groups) - it's a moderately activating group. The ketone is attached via the carbonyl group which is deactivating due to inductive and resonance effects.. So the reactivity:
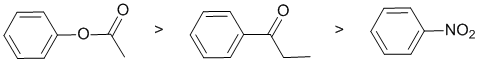
Qu3:
Acidity...Remember the lower the pKa the stronger the acid, and think of the simple acid equation HA <=> H+ A- then look for factors that stabilise A-.... Here we are looking at -OH systems in a series of phenols (i.e. aromatic alcohols). Phenol itself is the simplest system which has a pKa = 10. We also have p-hydroxybenzoic acid which is a carboxylic acid where there is extra resonance to a second electronegative O atom (pKa approx. 5). Our third phenol is p-cyanophenol and the cyano group is strongly electron withdrawing due to the electronegative N atom which will stabilise the conjugate base compared to simple phenol via resonance (but not to the same extent as in a carboxylic acid). So in terms of acidity:
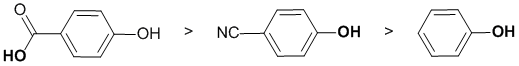
Qu4:
The reactivity of C=C pi bonds towards sulfuric acid so we are looking at electrophilic addition to an alkene which will be controlled by the stability of the intermediate carbocation (more stable C+ forms faster in the rate determining step). A closer look at the alkene structures shows that each of the alkenes has a different substituent attached: a chlorine, an alkyl group -R, and an alkoxy group -OR. Each alkene will react with the acid to give a carbocation and the substituent will affect the carbocation stability.... an electron donor will stabilise a carbocation, an electron withdrawer will destabilise a carbocation. Looking at the substituents: a chlorine is weakly electron withdrawing, an alkyl group, -R, is a weak electron donor, and an alkoxy group, -OR, is a strong electron donor due to resonance. So in terms of reactivity of the alkenes towards H+ :
![]()
Qu5:
Acidity...Remember the lower the pKa the stronger the acid, and think of the simple acid equation HA <=> H+ A- then look for factors that stabilise A-.... Here we are looking at carbonyl systems and the formation of enolates by the removal of H from the C atom adjacent to the C=O (known as the alpha position) since this allows for resonance stabilisation of the -ve charge. In a simple ketone, such as cyclohexanone, the pKa is about 20, in an aldehyde the pKa is about 17. Finally, benzaldehyde which is not just a simple alkyl aldehyde because benzaldehyde does not have alpha-H and is therefore less acidic than the other carbonyl systems.
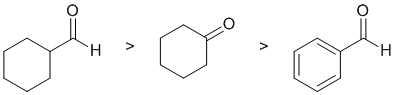
Qu6:
This question relates to
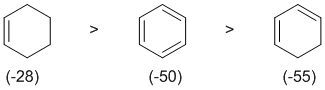
resonance energy and aromaticity. Aromatic systems have high resonance stablisation and therefore high resonance energies. Conjugated systems also have resonance stabilisation but not to the same extent as aromatics. These 3 systems were discussed when we discussed how to evaluate resonance energy and the aromaticity of benzene (see the figure in the link above). The heats of hydrogenation (in kcal/mol) are cyclohexene = -28.6, 1,3-cyclohexadiene = -55.2 and benzene = -49.8. Knowing that the resonance energy of a conjugated diene is about 3 ~ 4 kcal/mol and benzene about 36 kcal/mol provides enough information to deduce the relative heats of hydrogenation.
Qu7:
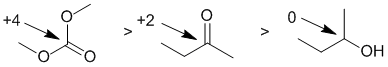
Oxidation states...The easiest way to reach the answer is based on the knowledge that ketones can be reduced to secondary alcohols. The other structure is a carbonate, a special type of ester. More bonds to O typically means a higher oxidation state. More formally, we count the bonds attached to the atom being considered. A bond to a more electronegative atoms (e.g. O) counts -1, a bond to the same type of atom counts 0 and a bond to a less electronegative atom (e.g. H) counts +1. Total the count and then consider the formal charge on the central atom since the oxidation state for the central atom plus the groups attached must equal the atoms formal charge. For the ketone, the C is attached to 2 x O (count - 1 each, total -2) and 2 x C (count 0) therefore total = -2 and therefore the oxidation state C = +2. In the secondary alcohol, the C is attached to 1 x O (count -1), 2 x C (count 0) and 1 x H (count +1) therefore total = 0 and therefore the oxidation state C = 0. In the carbonate ester the C is attached to 4 x O (count -4) therefore total = -4 and therefore the oxidation state C = +4.
Qu8:
First draw out the starting material and identify the reaction... alkene hydration. The issue is the regioselectivity. Aqueous acid proceeds via a carbocation intermediate via protonation of the C=C which is this case is going to lead to two different secondary C+, one of which will rearrange via a hydride shift to give a tertiary C+. This means a low yield of the desired alcohol. The other options are hydroboration / oxidations which are controlled by sterics and electronic effects. The desired alcohol is the less hindered isomer and therefore with the favoured most in the reaction of 9-BBN.
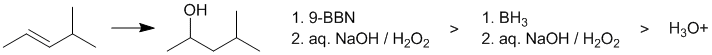
Qu9:
The reactivity of carbonyl groups of an aldehyde, an amide and a ketone towards sodium borohydride The electronic factors of each of the substituents on each of the carbonyl groups need to be considered. In the aldehyde we have a neutral H atom (i.e. no electronic effect). In the amide, the NR2 group, is a strong electron donating group. In the ketone, the -R group (an alkyl group) is a weak electron donors. Electron donors on the carbonyl make the carbon less electrophilic and less reactive. Hence in terms of reactivity:
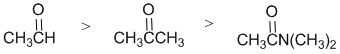
Qu10:
All about enantiomeric excess and optical rotation. With the masses of the two enantiomers drawn, the e.e. is 20% (R,R) (use (0.6 - 04)/(0.6 + 0.4)) hence rotation will be 20% of 12.7 = 2.54 degrees. For a sample of 1g in 10mL the rotation... [a]D (sample) = a / cl = +0.635 / (1.0/10) = 6.35. And for 1g sample of the meso isomer, the specific rotation = 0.
v1 ii > i > iii, v2 iii > i > ii.
STRUCTURES and PROPERTIES:
You need to know about functional groups and reactions, and how to apply concepts related to structure such as stereochemistry, acidity/basicity and reactivity etc.
Qu11:
The phenol has a -OH (the others are CH systems) and the phenol will be the most acidic (pKa = 10)
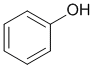
Qu12:
A yellow precipitate with 2,4-dinitrophenylhydrazine indicates the presence of an aldehyde or ketone and the yellow precipitate in the iodoform indicates a methyl ketone.
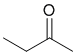
Qu13:
A yellow precipitate with 2,4-dinitrophenylhydrazine indicates the presence of an aldehyde or ketone and the silver mirror in the Tollen's test the presence of an aldehyde.
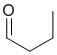
Qu14:
An orange solution with 2,4-dinitrophenylhydrazine indicates a negative test and means we don't have either an aldehyde or a ketone. If we have C=O as indicated in the question, then we have the ester.
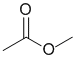
Qu15:
The highest H-NMR shift will be for the aldehyde H (9-10 ppm). Note that Ar-H are typically 7-8 ppm and phenol -OH are 3-7 ppm.

Qu16:
Look at the substituent effects on the aromatic ring... -OR groups are strongly electron donating due to the lone pairs on the atom adjacent to the ring (via resonance) so methoxybenzne is the most activated of these benzene systems.
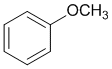
Qu17:
Look at the substituent effects on the aromatic ring... -NO2 groups are strongly electron withdrawing (induction and resonance) so nitrobenzene is the most deactivated of these benzene systems.
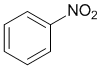
Qu18:
Look at the substituent effects on the aromatic ring... electron withdrawing groups are deactivating and direct meta and are characterised by the atom attached to the ring being formally or partially +ve as a result of several bonds to more electronegative atoms.
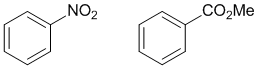
Qu19:
Friedel-Crafts alkylations only work on benzenes that are as active as monohalobenzenes or better so the halo-, alkyl- and alkoxy- benzenes...
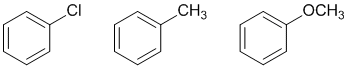
AROMATICITY and RESONANCE:
Best method is to work through the molecules and decide on the aromaticity based on the four criteria for the pi system (cyclic, planar, conjugated pi system with 4n+2 pi electrons).
Qu20:
To be non-aromatic as drawn, we need to find a system that fails on one of the first three criteria (i.e. it lacks a cyclic conjugated pi system) but has a resonance contributor that satisfies all four of the criteria for aromaticity (i.e. it has a pi system that is cyclic, planar, fully conjugated and contains 4n+2 pi electrons). The most likely scenario will involve an exocyclic alkene unit:
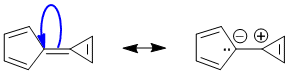
Qu21:
To be non-aromatic as drawn, we need to find a system that fails on one of the first three criteria (i.e. it lacks a cyclic conjugated pi system) but has a conjugate base that satisfies all four of the criteria for aromaticity (i.e. it has a pi system that is cyclic, planar, fully conjugated and contains 4n+2 pi electrons). The most likely scenario will involve loss of H+ from a sp3 center to create a conjugated lone pair (so adding 2 electrons to the pi system) ... for clarity the aromatic resonance structures of the conjugate bases are also shown:
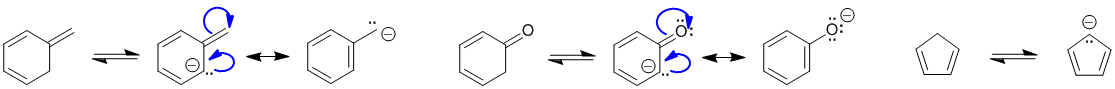
Qu22:
In a hydrocarbon, an isolated C=C will have sp3 C attached to each end.
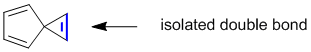
Qu23:
To be aromatic as drawn, we need to find a system that satisfies all four of the criteria for aromaticity (i.e. it has a pi system that is cyclic, planar, fully conjugated and contains 4n+2 pi electrons). Most of the systems don't have cyclic conjugated pi systems, only the 3 shown in blue do. Then the pi electron count is used to establish the aromaticity....
Qu24:
To be non-aromatic as drawn, we need to find a system that fails on one of the first three criteria (i.e. it lacks a cyclic conjugated pi system) but will need to be able to rearrange (e.g. relocate a double bond, tautomerise) to give a product that is aromatic and satisfies all four of the criteria for aromaticity (i.e. it has a pi system that is cyclic, planar, fully conjugated and contains 4n+2 pi electrons).
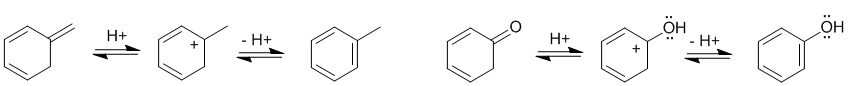
Qu25:
The most common form of tautomerism switches H and double bond positions... To be non-aromatic as drawn, we need to find a system that fails on one of the first three criteria (i.e. it lacks a cyclic conjugated pi system) but has a tautomer that satisfies all four of the criteria for aromaticity (i.e. it has a pi system that is cyclic, planar, fully conjugated and contains 4n+2 pi electrons):
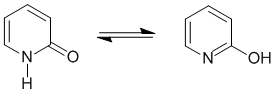
Qu26:
The availability of the electrons is the key factor.... if a lone pair on a heteratom is initially part of the aromatic pi system and those electrons are then used to make the new bond to the H+ and results in a loss of aromaticity (and hence stability) then those electrons are less available and we have a weaker base
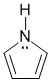
Qu27:
The availability of the electrons is the key factor.... hybridisation of the orbital containing the lone pair and resonance are key factors. Lone pairs in sp3 orbitals are further from the positive nucleus and are more available and more basic.
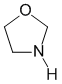
Qu28:
The N atom will need to have 3 sigma bonds and be adjacent to a pi system.
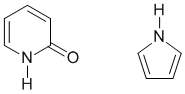
Qu29:
Resonance energy is the stabilisation that results from the interaction of pi systems that are conjugated. Aromatic compounds have high resonance energy and more double bonds in the aromatic system will mean a higher resonance energy.
STARTING MATERIALS AND PRODUCTS:
If you are trying to find the product, then you should probably just work forwards through the sequence of reactions.
If you are looking for the starting material, then working backwards is probably the best way to go....
Basically depends on the need to know and identify the reactions, this is often triggered by looking at the functional groups in the molecules.
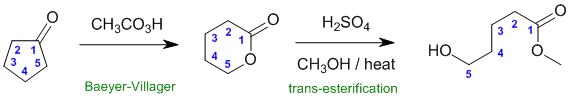
Qu30:
Working backwards,
the product is a methyl ester.... step 1 is a peracid, possibly used for either epoxidation of an alkene or the Baeyer-Villager reaction of a ketone to give an ester which is the best fit here. Count the C atoms in the ester backbone to match the appropriate cyclic ketone.
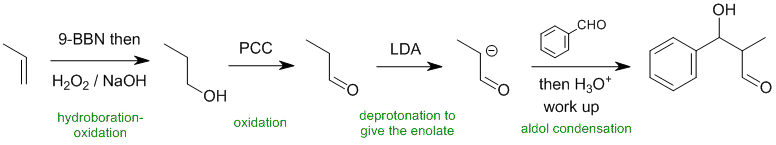
Qu31:
Working forwards, the alkene undergoes hydroboration / oxidation with 9-BBN (9-borabicyclononane) give the anti-Markovnikov product, the primary alcohol. The alcohol is then oxidised with PCC (pyridinium chlorochromate) to give the aldehyde. The aldehyde is then reacted with a base, LDA (lithium diisopropylamide) to form the enolate and then reacted with benzaldehyde in an aldol reaction.
Qu32:
Working forwards, step 1 is a mCPBA (m-chloroperbenzoic acid) used for epoxidation of the alkene. Reaction of a Grignard reagent with the epoxide at the least hindered end gives the alcohol to give the alcohol trans to the methyl group. The resulting alcohol is then converted into an ether via an SN2 reaction, a Williamson ether synthesis.
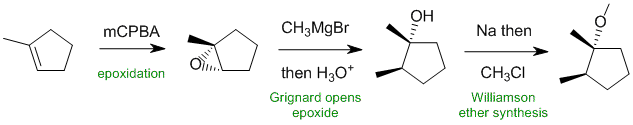
Qu33:
Working forwards....Friedel-Crafts acylation of ethylbenzene gives the methyl ketone para to the alkyl group. Wolff-Kischner reduction converts the C=O to a CH2 which then undergoes chlorination via electrophilic aromatic substitution.
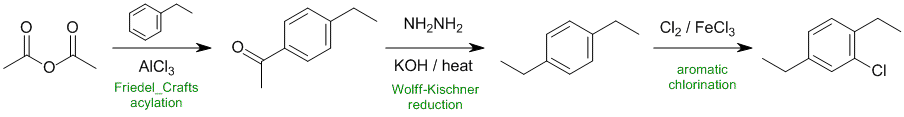
Qu34:
Working backwards....The most obvious feature are the two carbonyl groups and the ozonolysis with oxidative work up. Reconnecting the two carbonyls reveals a bicyclic structure that suggests a Diels-Alder reaction for which we need to find the dienophile.
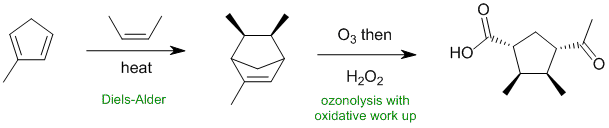
Qu35:
Working forwards, step 1 is a peracid used for epoxidation of the alkene. Hydrolysis of the epoxide creates a 1,2-diol (with overall anti- stereochemistry from the alkene). The 1,2-diol reacts with the aldehyde, butanal, to give a cyclic acetal.
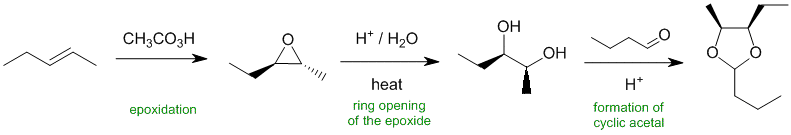
Qu36:
Working forwards.... bromobenzene reacts with the Mg to form the Grignard reagent which is reacted with CO2 to give the carboxylic acid. The carboxylic acid is then converted to the more reactive acyl halide and then tine amide. Reduction of the amide gives the amine.
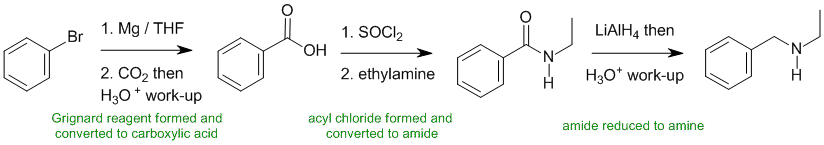
Qu37:
Working backwards....looking and the product and the first step, we need to make a benzene ring. The first step looks to involve a Friedel-Crafts reaction which would mean using one of an alkyl halide, a acyl halide or an anhydride... we only have one of those as a choice. Therefore we have a Friedel-Crafts
acylation to give the ketone. The ring is formed by a second Friedel-Crafts
acylation using the acyl chloride. Reduction of the carbonyls to the alcohols and then dehydration readily yields the naphthalene system.
Qu38:
Working backwards.... the product is a carboxylic acid which looks to have been made by the hydrolysis of a nitrile which was made via a substitution of a primary bromide. The bromide was formed from an anti-Markovnikov addition (radical) to an alkene.
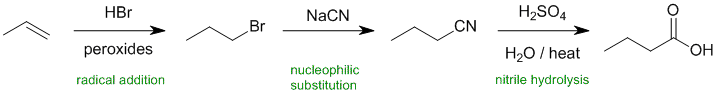
REAGENTS FOR SYNTHESIS:
Need to be able to look at reactions, looking at the functional groups in the starting materials and products of each step to think about
how you have got there.
How well do you know your reagents
? Look at what has actually happened in terms of the reaction functional
group transformation and then first look for any regiochemical issues
then finally the stereochemistry last (it's the hardest to sort out).
Qu39:
The product is a halohydrin which are typically formed via an anti addition. Analysis of the stereochemistry of the Fischer projection indicates that in order to get the right stereochemistry in the final product, the anti addition will need to occur on a trans C=C formed from the alkyne. Therefore need to use i. Na / NH3 then ii. Br2/ H2O.
Qu40:
The product has 2 more methyl groups than the starting material, thus, oxidation of the primary alcohol to the carboxylic acid and then conversion to the ester will allow 2 equivalents of a Grignard reagent to be used to obtain the tertiary alcohol. Therefore we need to: i. K2Cr2O7 / H2SO4 / H2O ii. TsOH / methanol iii. MeMgBr (excess) then H3O+
Qu41:
Need to alkylate the terminal alkyne adding two carbon atoms but the presence of the ketone means the alpha-H in the methyl group adjacent to the ketone are more acidic H than the terminal alkyne and therefore the ketone needs to be protected first as the ketal. So use i H+ / HOCH2CH2OH, ii. NaNH2, iii. CH3CH2Br, iv. H+ / H2O / heat.
Qu42:
Halogens are deactivating but direction o,p. The required product is meta. Therefore we should use diazonium chemistry to get the right regiochemistry. So nitrate, then add a halogen before converting the nitro to the amine and then the diazonium group before substituting the other halide. Therefore: i. HNO3 / H2SO4, ii. Cl2 / FeCl3, iii. Sn / HCl then aq. NaOH, iv. NaNO2 / aq. HCl / cold then CuBr
Qu43:
Looking at the product we need to get a p-dicarboxylic acid. The viable route involves double Friedel-Crafts alkylation followed by oxidation of the benzylic postions: i. 2-chloropropane (2 equivalents) / AlCl3, ii. Na2Cr2O7 / H2SO4 / H2O / heat
Qu44:
The product is a ketone which can be formed by oxidation of secondary alcohols which can be formed by adding organometallic reagents to aldehydes. In this case, the acetylide has been made by utilising a simple Grignard reagent as a base. i. MeMgBr, ii. benzaldehyde then H3O+ work-up, iii. PCC
EXPLANATION OF PHENOMENA
Qu45:
The reaction is the acid catalysed addition of H2O to the alkene which is controlled by the stability of the carbocation intermediate produced when H+ adds to the C=C. In this case favouring a resonance stabilised benzylic carbocation. This means the product is the benzylic alcohol. Since there are the same number of groups attached to each end of the alkene, Markovnikov's empirical rule doesn't help us here.
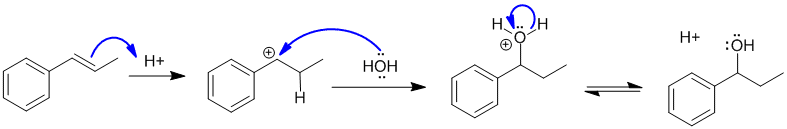
Qu46:
The reaction is the normal (cation) addition of HBr to the alkene which is controlled by the stability of the carbocation intermediate produced when H+ adds to the C=C. In this case the addition occurs to the more electron rich C=C to give the more stable tertiary carbocation. This means the product is the tertiary bromide.
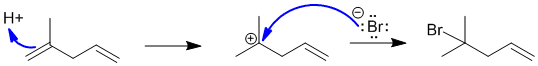
Qu47:
It's the group on the ring that directs the substitution, deactivators direct meta. The nitro group, -NO2, is strongly deactivating and a m-director.
Qu48:
In the more acidic amide, there is at least one H attached to the N atom (remember that N is electronegative and can stabilise the conjugate base). In the other amide, the N has only C atoms attached and the most acidic H is alpha to the carbonyl group.
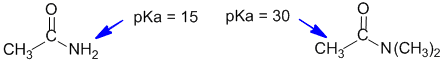
Qu49:
The reaction shows the addition of HCl to the alkene of a conjugated diene giving the product from the reaction of the chloride ion at the more stable tertiary carbocation. This means the product is the tertiary chloride and corresponds to the kinetic product which is favoured at lower temperatures.