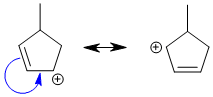Here is an post-mortem analysis / "how to" for the MT. The questions are split by the sections. At the start of each section are a few suggestions of what to look for or how to tackle the question type.
RELATIVE PROPERTIES:
Identify the controlling feature, which is not
always as obvious as it may appear. Look for two pairs of similar systems to compare
that have minimal differences in structure. If a compound is named, draw it out.
If a reaction is involved, identify the type of reaction and then what the controlling
factors are.
Qu1:
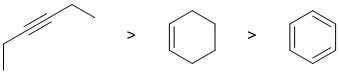
Alkenes undergo electrophilic addition. In the reactions with HCl, the rate is controlled by the rate of carbocation formation. In this case, this means the more stable the carbocation, the faster the reaction. The alkene will react the faster (via secondary carbocation) than the alkyne which are less reactive since they would require the much less favourable termolecular pathway to avoid the formation of the very unfavourable vinyl carbocations. The ease of carbocation formation dictates the rate of reaction. In the case of alkynes, the vinyl C+ would be too unstable and a slower termolecular mechanism occurs, so the alkyne is the slowest. Arene C=C don't react with HCl.

Qu2:
Use the approx pKas of the acids: the alkyne is not terminal, so the CH is likely about 45, amine NH = 35 and alcohol = 15. The electronegativity of the atom the acidic H is attached to is a key factor. Remember the question wants in acidity order (not pKa order), the most acidic H in each structure is shown in bold.
![]()
Qu3:
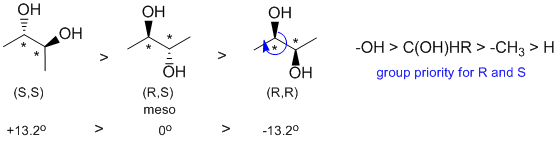
All about specific rotation. Looking at the three structures, we need to use the Cahn-Ingold-Prelog rules to assign the configurations. One is (R,R), one is (S,S) and the other is (R,S), a meso compound and therefore is optically inactive and has by definition a specific rotation = zero. Given the data in the question, the (S,S) has specific rotation of +13.2 degrees.
Qu4:
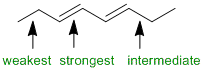
The relative stability of systems.... in this case we are looking at dienes and alkynes and the degree of alkene substitution. Conjugated dienes are more stable than isolated dienes, and isolated alkenes tend to be more stable than alkynes (which are much more like cumulated dienes).
![]()
Qu5:
All the bonds are CC bonds. The factors involved are (1) the hybridisation of the C atoms involved and (2) the bond order. Double bonds are stronger than single bonds. Bonds involving sp2 and sp3 C atoms are stronger than two sp3 C atoms (due to higher s character).
Qu6:
The question is about the hydroboration / oxidation of alkynes (which is the anti-Markovnikov product) with gives a C=O group (aldehyde or ketone). The symmetric alkyne reaction does not have regiochemistry and the product must be a methyl ketone. For the non-symmetrical cases, increasing the size of the alkyl groups will increase the selectivity of the reaction, increasing the yield of the methyl ketone (via B group at the least hindered end of the alkyne).
![]()
Qu7:
The reaction is a catalytic hydrogenation. The reaction is fastest for the weakest pi bond and that is found in an alkyne... so the alkyne is the most reactive. In contrast, the C=C in arenes are less reactive than those in alkenes (alkenes can be reduced in the presence of arenes).
Qu8:
Carbocation stability.... Look at the C atom bearing the charge and what's attached to it. We have a tertiary allylic carbocation, a secondary allylic and a simple secondary carbocation. The simple secondary carbocation is the least stable and the allylic carbocations are more stable since they are resonance stabilised by a C=C. The tertiary allylic is more stable than the secondary allylic carbocation.
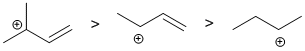
Qu9:
The question is about the The hydroboration / oxidation reactions of 1-cyclohexylprop-1-ene to give an alcohol. The reaction adds the HO and H across the C=C in an anti-Markovnikov fashion. Steric effects are the important factor here. Note that the primary alcohol would not be expected to form given the location of the original alkene.
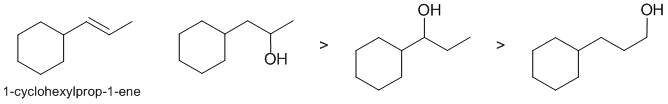
Qu10:
The question is about the hydration reactions of hex-1-ene to give hexan-1-ol (which is the anti-Markovnikov product). aq. H2SO4 will give hexan-2-ol as the major product (Markovnikov product). The hydroboration / oxidation of alkenes adds the HO and H across the C=C in an anti-Markovnikov fashion and 9-BBN tends to show enhanced selectivity due to increased steric effects. Therefore is terms of yields of the hexan-1-ol: 9-BBN > BH3 > H3O+
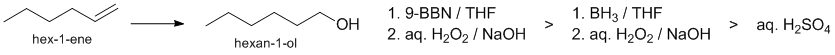
STARTING MATERIALS, REAGENTS AND PRODUCTS:
If you are trying to find the product, then
you should probably just work forwards through the sequence of reactions.
If you are looking for the starting material,
then working backwards is probably the best way to go....
Basically depends on the need to know and identify
the reactions, this is often triggered by looking at the functional groups in
the molecules.
Qu11:
Working backwards : the products (two aldehydes) are from the ozonolysis with a reductive work up of an alkene formed by the acid catalysed dehydration of an alcohol. Count C atoms.

Qu12:
Working backwards : MCPBA has been used to prepare the epoxide from the alkene made by the elimination of an alkyl halide.
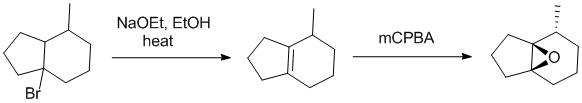
Qu13:
Working forwards : the terminal alkyne is catalytically reduced to the alkene which then undergoes a radical (anti-Markovnikov) addition of HBr.
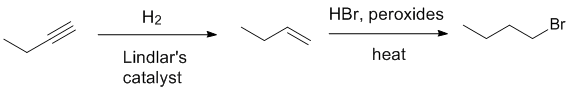
Qu14:
Working backwards : the product is a halohydrin which will have been formed from an alkene that was in turn generated by an elimination of an alkyl halide that was the product of a benzylic radical halogenation.
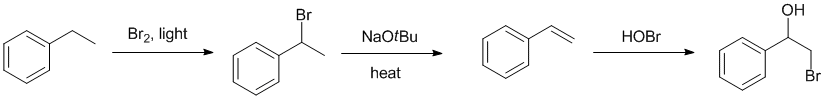
Qu15:
Working forwards : formation of a dibromide via addition of Br2 to the alkene and then a SN reaction of a reactive benzylic position.
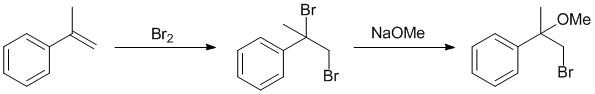
Qu16:
Working backwards : the products are consistent with the ozonolysis of a terminal alkyne that was formed via the double elimination of the 1,1-dibromide.
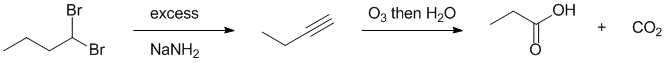
Qu17:
Working forwards : hydroboration / oxidation of alkenes give the anti-Markovnikov alcohol via a syn addition (concerted reaction). This is converted to the tosylate with the same stereochemistry and then an E2 elimination where the 180 degree requirement forces an anti-Zaitsev elimination.
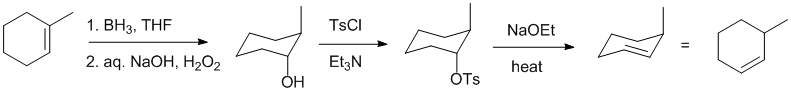
Qu18:
Working forwards : aq H2SO4 will cause hydration of the alkene via a carbocation that will have undergone a rearrangement (secondary carbocation to more stable tertiary carbocation via a 1,2-hydride shift).
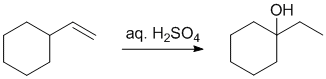
REGIO- and STEREOCHEMISTRY:
How well do you know your reagents ? Look
at what has actually happened in terms of the reaction functional group transformation
and then first look for any regiochemical issues then finally the stereochemistry
last (it's the hardest to sort out). In cases where more than one product
is formed in equal amounts (e.g. the enantiomers), then both must be selected
for full marks, part marks are given when only one of the pair is selected.
Advice : in each case draw the starting material in the
conformation in which is reacts or the product in the conformation in which
it is initially formed using wedge-hash diagrams. It is a good idea to draw
the materials in such a way that the new bonds are in the plane of the page.
Once you have drawn the materials it may also be good for you to use model kits
for these questions too. Once you have drawn the materials in this way,
you may need to consider rotations around sigma bonds to make your answer match
the options. An alternative approach could be to assign configurations
to your drawn answer to compare them with the options - this can be slow and
prone to error.
Qu19:
Since the addition of Br2 to an alkene occurs with anti stereoselectivity, the alkene required will need to be trans. Trans alkenes are the products of dissolving metal reductions of alkynes.
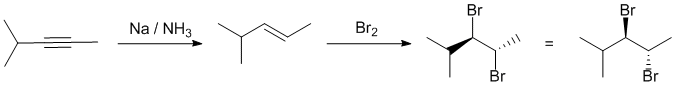
Qu20:
The Mg will react with the aryl bromide to form the Grignard reagent which is a strong nucleophile. A strong Nu will react at the least hindered end of the epoxide in an SN2 fashion causing the epoxide to open. The stereochemistry at the alcohol center is defined by the stereochemistry of the epoxide starting material.
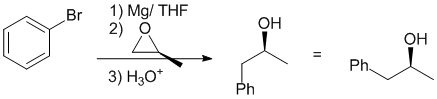
Qu21:
Working forwards : deprotonation and methylation of the terminal alkyne is followed by catalytic reduction to the cis-alkene. Dihydroxylation using aq. basic KMnO4 is used to make the 1,2-diol via an syn addition.
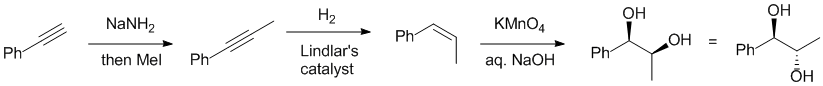
Qu22:
Working forwards : the alkyne undergoes catalytic reduction to the cis-alkene. Peracetic acid forms the epoxide with the same stereochemistry and then the epoxide is opened using a weak nucleophile with an acid catalyst. This means the methanol reacts at the end of the epoxide best able to accommodate partial +ve charge (regiochemistry) but with inversion at the site of attack (stereochemistry). It's best to visualise the ring opening of the epoxide with a wedge hash diagram (or a model kit) and then convert that to a Fischer projection (remember to position the alkyl chain (vertical) and back into the page with all the horizontal bonds coming out towards you).
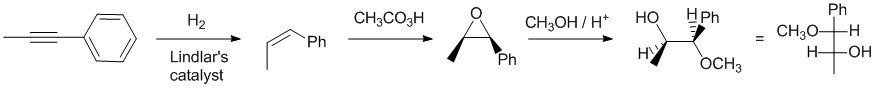
Qu23:
Working forwards : the alkyne undergoes dissolving metal reduction to the trans-alkene. Oxymercuration / demercuration will give the alcohol at the C best able to accommodate the partial +ve charge that develops during the reaction (at the benzylic position).
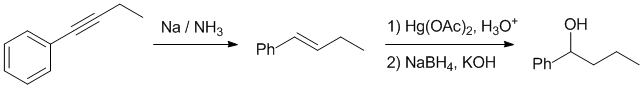
Qu24:
Working backwards : the alcohol product is formed via a hydroboration / oxidation of an alkene (anti-Markovnikov, syn addition). The alkene was formed via the elimination of an alkyl bromide via an E2 pathway which would require the H-C and C-Br bonds to be anti (180 degrees). One also needs to consider if the elimination gives a mixture of alkenes or one in order to get the best answer.
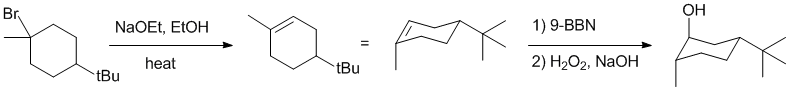
Qu25:
Working forwards : the halohydrin reacting with Na2CO3 (a weak base) to make an epoxide via an SN2 reaction so we need to make sure we review the halohydrin in the reactive conformation with the O nucleophile undergoing a backside attack to the C-Cl bond.
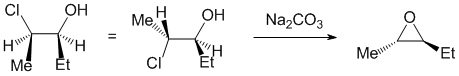
PI SYSTEMS:
All about knowing properties of pi systems such as resonance, structure, bonding, shapes, and terminologies etc.
Qu26:
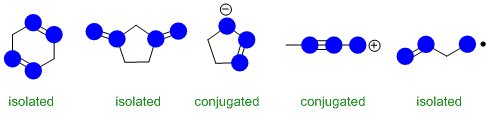
Conjugation requires an extended pi system (at least 3 atoms) where the pi systems can interact
(parallel orbitals, not perpendicular). The image below has highlighted the p orbitals (blue circles for top down view of vertical p orbitals.
Qu27:
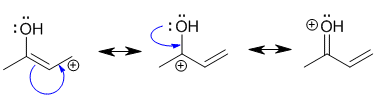
Resonance contributors are derived via the delocalisation of the pi electrons across the pi system and can be derived by pushing curly arrows. Use the rules for recognising resonance structures.
Qu28:
Alkenes undergo electrophilic addition. In the reactions with HCl, the rate is controlled by the rate of carbocation formation with the most stable carbocation forming the fastest. The most stable carbocation will be the tertiary benzylic carbocation.

Qu29:
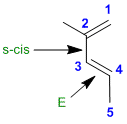
The key issues are (1) conjugated systems are more stable than isolated systems, (2) alkyl groups on pi bonds stabilise them, (3) trans isomer are more stable than cis isomers (steric effects), (4) s-trans is typically more stable than s-cis. Therefore the most stable isomer is the s-trans-trans,trans-conjugated diene.
Qu30:
Qu31:
Tautomers (remember alkyne hydration ?) of ketones are the enols.
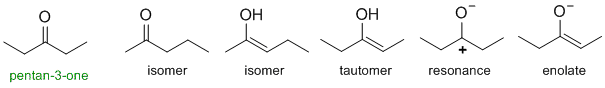
Qu32:
The addition of HBr / uv light to an alkene is a stepwise process with Br radical adding to form a radical intermediate:
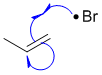
Qu33:
Organic cis / trans nomenclature is based on the stereochemistry of the chain the defines the root name.
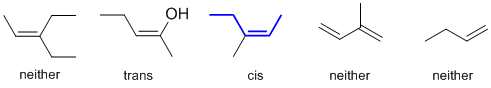
Qu34:
Resonance contributors are derived via the delocalisation of the pi electrons across the pi system and can be derived by pushing curly arrows. Use the rules for recognising resonance structures.