Here is an post-mortem analysis / "how to" for the MT. The questions are split by the sections. At the start of each section are a few suggestions of what to look for or how to tackle the question type.
RELATIVE PROPERTIES:
Identify the controlling feature, which is not
always as obvious as it may appear. Look for two pairs of similar systems to compare
that have minimal differences in structure. If a compound is named, draw it out.
If a reaction is involved, identify the type of reaction and then what the controlling
factors are.
Qu1: C
Carbocation
stability.... due to (a) alkyl groups, which are weak electrons donors, and (b) resonance with a C=C pi system. These effects add stability due to charge delocalisation. i is secondary and allylic, ii is also secondary and allylic, but here the other contributor is tertiary (hence this system is more stable than i) and iii is secondary with no resonance stabilisation (the sp3 center prevents the resonance). This means
that the simple secondary cation in i is the least stable of the three.
The allylic resonance in ii to the tertiary center makes it more stable than i. Hence ii > i > iii.
Qu2: C
All the bonds are CH bonds. The factors involved are (1) the hybridisation of the C atom (2) primary vs secondary and (3) allylic systems. In terms of hybridisation, sp3 C-H bonds are weaker then sp2 C-H due to the increased s character in the sp2 situation creating a shorter and stronger bond. Primary CH bonds are stronger than secondary C-H bonds (due to the weak electron donating effect of alkyl groups). Allylic C-H bonds are weaker due to the proximity of the electron rich pi bond.
In i we have a primary sp3 system that is allylic. In ii is a vinyl C-H i.e. sp2C. In iii we gave a secondary sp3 system that is allylic. So in terms of C-H bond strengths: ii > i > iii.
Qu3: D
Reaction
is about additions of HX to alkenes or alkynes. These reactions give the Markovnikov product via the most stable carbocation intermediate. The rate of the reaction
is controlled by the formation of the carbocation and the variable here is the pi system and hence the carbocation intermediate. i is benzene and as such the C=C present in benzene do not react like those of an alkene (benzene does not react with HCl) ii is an alkene which reacts to give a simple alkyl carbocation while iii is an alkyne. Alkynes are less
reactive since they "require" the formation of the less stable vinyl carbocations. So ii > iii > i.
Qu4:E
Reaction
is about additions of HX to an alkene. This gives the Markovnikov product via the most stable carbocation intermediate. The rate of the reaction
is controlled by the formation of the carbocation and the variable here is the pi system and hence the stability of the carbocation intermediate. i would form a tertiary carbocation. ii would form a secondary carbocation. iii would form a tertiary, benzylic carbocation. Since tertiary carbocations are more stable than secondary and benzylic cations are further stabilised by resonance, the reactions proceed such that iii > i > ii.
Qu5: A
In this question we are looking at types of polyenes (dienes) and the
degree of substitution. i and ii are both conjugated dienes whereas iii is an isolated diene so ii and iii are more stable than i. The difference between i and ii is that i has
a tri and a disubstituted C=C while ii has two disubstituted C=C, so i is more stable than ii. Therefore we get i > ii > iii in terms of stability.
Qu6: C
All about enantiomeric excess and optical rotation. For i based on
the masses of the two enantiomers drawn, the e.e. is 50% (use (0.75 - 0.25)/(0.75
+ 0.25)). For ii a little more complex, but work out the e.e. via the
rotation... [a]D (sample) = a / cl = +2.54 / (2.0/10) = 12.7. Therefore e.e. = 100%. And for iii a racemic mixture has an e.e. of 0 (by definition). So ii > i > iii.
Qu7: A
The reaction is the radical addition of HBr to alkenes. First draw out the starting material, 2-methyl-2-pentene. i and ii have correctly added the Br and H across the C=C therefore iii has the lowest yield. ii is the Markovnikov product, while iii is the anti-Markovnikov product. Since the radical addition of HBr to alkenes gives the anti-Markovnikov product as the major product then we get i > ii > iii.
Qu8: C
Experimental yields.... First you need to balance the reaction equation and then work out the moles of each reagent used to determine the limiting reagent:
3 CH3CH=CH2 + BH3--[work up] --> 3 CH3CH2CH2OH |
|||
propene |
borane |
propan-1-ol |
|
| MW (g/mol) | 42 |
15 |
60 |
i 4.2 g propene = 0.1 mol, due to the stoichiometry there is excess borane so the alkene is the limiting reagent and we can get 3 x 0.1 mol of alcohol. We get 3.0g of alcohol = 0.05 mol, so yield = 0.05 / 0.1= 50%
ii 4.2 g propene = 0.1 mol, due to the stoichiometry borane is the limiting reagent (0.02 mol) and we can get 3 x 0.02 mol of alcohol. We get 3.0g of alcohol = 0.05 mol, so yield = 0.05 / 3 x 0.02 = 83%
iii propene = 0.3 mol, due to the stoichiometry borane (0.1 mol) either alkene or borane and we can get 0.3 mol of alcohol. We get 6.0g of alcohol = 0.1 mol, so yield = 0.1 / 0.3 = 33%
So ii > i > iii.
Qu9: AB
Reaction is the Diels-Alder reaction and we are looking at the dienophiles reacting with cyclopentadiene.... The reactivity increases in the normal Diels-Alder reaction with electron withdrawing groups on the dienophile. i has an alkyl group (a weak electron donor), ii has an electron withdrawing carbonyl in the form of an ester and iii has two electron withdrawing carbonyl groups in the form of a cyclic anhydride. Therefore the reactivity is iii > ii > i.
Qu10: AB
Oxidation states can be calculated or more simply assessed by counting C-O bonds or we can look at the chemical relationship of the compounds. As we look at the 3 compounds, i is an alkene, ii is a diol and iii is a dicarboxylic acid. For the C atoms that are changing, the acid system has 3 CO bonds per C, the diol has 1 CO bond and the alkene none, so in terms of oxidation state, iii > ii > i. If we think about reactions, the diol ii is made by the oxidation and the diacid iii is made via ozonolysis with oxidative work-up of the alkene i.
STARTING MATERIALS AND PRODUCTS:
If you are trying to find the product, then
you should probably just work forwards through the sequence of reactions.
If you are looking for the starting material,
then working backwards is probably the best way to go....
Basically depends on the need to know and identify
the reactions, this is often triggered by looking at the functional groups in
the molecules.
Qu11: A
Working forwards... the alkyne reacts with the sodium amide to form the nucleophilic terminal acetylide which then reacts with the epoxide in a by basic ring opening to give a primary alcohol / alkyne, HC≡CCH2CH2OH. The Markovnikov hydration
of the terminal alkyne then gives a methyl ketone, A.
Qu12: E
Overall we need to convert the alkyne into a cyclopropyl system. Cyclopropanes are made from alkenes via carbenes. Since the methyl groups on the cyclopropane are cis, we need a cis-alkene which can be made via the catalytic
hydrogenation reduction of alkyne to an alkene using Lindlar's catalyst (C or E). In order to add the CBr2 unit we can't use the Simmonds-Smith reaction (these would give a CH2 unit), we need to use the HCBr3 / strong base route to give the right carbene, so E.
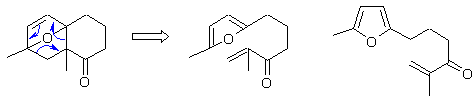
Qu13: E
An intramolecular Diels-Alder reaction with a furan diene.... work backwards from the product... locate the cyclohexene unit and push the arrows backwards to reveal the starting material.

Qu14: E
Working forwards... the catalytic
hydrogenation (a reduction) of an alkyne to an alkene ,1-butene, CH3CH2CH=CH2 using Lindlar's catalyst of the alkyne followed by addition of HBr to the alkene giving the Markovnikov product, 2-bromobutane, CH3CHBrCH2CH3.
Qu15: D
Working backwards...reactions look like alkene ozonolysis with a reductive work-up and therefore step 1 has formed the alkene via an E2 elimination of the alkyl halide. To identify the alkene, locate the two C=O groups in the product and reconnect them to reveal the 5 membered ring (numbering the C atomsfrom the aldehyde helps) ethylcyclopentene which is formed from D. Simple alcohols do not eliminate using basic reactions conditions... this rules out B and C.
Qu16: B
Working forwards..... this is hydration of the alkene to give an alcohol following Markovnikov's rule, but remembering to account for the carbocation rearrangement of the initial secondary carbocation to the more stable tertiary carbocation via a 1,2-methyl shift. C has ignored the alkyl shift.
Qu17: A
Working forwards..... the reaction is the hydroboration-oxidation
of an alkene to give an alcohol (anti-Markovnikov).
Qu18: E
Working forwards..... the alkene will react with the concentrated acid to protonate to give the more stable carbocation then the initial secondary carbocation to the more stable tertiary carbocation via a 1,2-methyl shift. Since there is no nucleophile present, the carbocation can loose a proton (just like in the last step of an E1 reaction) to give the more stable alkene, E.
REGIO- and STEREOCHEMISTRY:
How well do you know your reagents ? Look
at what has actually happened in terms of the reaction functional group transformation
and then first look for any regiochemical issues then finally the stereochemistry
last (it's the hardest to sort out). In cases where more than one product
is formed in equal amounts (e.g. the enantiomers), then both must be selected
for full marks, part marks are given when only one of the pair is selected.
Advice : in each case draw the starting material in the
conformation in which is reacts or the product in the conformation in which
it is initially formed using wedge-hash diagrams. It is a good idea to draw
the materials in such a way that the new bonds are in the plane of the page.
Once you have drawn the materials it may also be good for you to use model kits
for these questions too. Once you have drawn the materials in this way,
you may need to consider rotations around sigma bonds to make your answer match
the options. An alternative approach could be to assign configurations
to your drawn answer to compare them with the options - this can be slow and
prone to error.
Qu19: D
Working forwards...the starting material is an alkyne, the first set of reagents indicate
a dissolving
metal reduction of an alkyne to the trans-alkene. Next oxymercuration / demercuration of an alkene gives the alcohol (Markovnikov product). In terms of regiochemistry, the water nucleophile will attack the cyclic mercurinium ion at the benzylic position due to the cation character.
Qu20: B
Working forwards... we have the reaction of an alkene
with a halogen in the presence of water gives a 1,2-halohydrin via a cyclic
halonium ion, the regiochemistry will put the -OH at the more substituted
position with anti stereochemistry to the Br atom and the -OH group. Treatment with potassium hydroxide as a base forms the epoxide with the same stereochemistry as the original alkene. This is followed by acidic ring opening using methanol (note the -OH and -OMe groups in the product). This means that the reaction is SN1 like and the methanol attacks the more substituted C in the epoxide.... hence we end up with trans-hydroxy and methoxy groups via an anti type ring opening. Note the D has the wrong stereochemistry. A, C and E have the wrong functional groups.
Qu21: D
Looking at the reactions, we have the hydroboration-oxidation
of an alkene followed by a Williamson ether synthesis (note the cyclic ether in the product). The hydroboration reaction gives the
anti-Markovnikov alcohol via a syn addition due to the concerted addition of
the B and H across the C=C. The product ether will have the same stereochemistry as the alcohol because the oxygen of the alcohol functions as the nucleophile. In terms of regiochemistry, the -OH is added at the least hindered end of the alkene (i.e. the methyl group end). A and C have too many methyl groups, B would form a diol and hence not lead to an ether and E the wrong substitution pattern.
Qu22: B
Looking at the reactions, we have the dihydroxylation
of an alkene to give a diol followed by a double dehydration of the alcohols to make a diene ready for a Diels-Alder reaction. Reversing the curly arrows in the cyclohexene reveals the dienophile (again) and the diene as 2-methylcyclopentene. So our original starting material needs to be methylcyclopentene, B.
Qu23: C
The first reaction is the catalytic
hydrogenation reduction of alkyne to an alkene which is a syn addition giving the cis-alkene. This then reacts with Br2 via a cyclic bromonium ion to give a 1,2-dibromide via an anti addition. The best approach (other than using models) is to imagine rotating the Newman projections to put the 2 Br atoms anti (as E already shows) and then look at the relative positions of the methyl and ethyl groups. A, B, and E each have the wrong alkene stereochemistry and D has the wrong regiochemistry of the 2 Br atoms. Alternatively, derive the answer using wedge-hash diagrams and then convert to a Newman projection then rotate each of the potential answers to see if it matches your answer.
Qu24: E
The first reaction is the catalytic
hydrogenation reduction of alkyne to an alkene which is a syn addition giving the cis-alkene. Then reaction
is about of HX to an alkene which gives the Markovnikov product via the most stable carbocation intermediate including an alkyl shift rearrangement to give the tertiary chloride. The strong bulky base will then eliminate the chloride via E2 elimination of the alkyl halide giving the anti-Zaitsev product.
Qu25: B
Working forwards... we have the reaction of N-bromosuccinimde (radical substitution) to give the benzylic bromide. Epoxidation of the alkene with the peracid gives an epoxide. Treatment of the bromo-epoxide with excess KCN causes SN2 like substitution of the epoxide at the least substituted end and SN2 substitution of the benzylic bromide to give a 2-hydroxy-1,3-dinitrile.... A, C, D, E all have the wrong regiochemistry.
PI SYSTEMS:
All about knowing properties of pi systems such as resonance, structure, bonding, shapes, and terminologies etc.
Qu26: AD
Conjugation requires an extended pi system (at least 3 atoms) where the pi systems can interact
(parallel, not perpendicular). This is not restricted to all carbon systems.
Make sure you draw out all the bonds to be sure. A is an isolated diene. B is a conjugated diene. C has is an allylic cation (and is therefore conjugated). D is a cumulated diene (i.e. perpendicular pi systems). E is a conjugated diene.
Qu27: AD
Resonance contributors are derived via the delocalisation of the pi electrons with the pi system and can be derived by pushing curly arrows. B can be ruled out because the +ve charge on the methyl group makes no sense. C and E are not contributors because they have extra sp3 C centers.
Qu28: A
The key facts here are (1) conjugated dienes are more stable than isolated dienes (due to conjugation stabilisation), (2) in acyclic systems, trans alkenes are more stable than cis (sterics) and (3) in conjugated dienes the s-trans is more stable than the s-cis (sterics). Therefore the most stable system is A where we have a conjugated diene with two trans double bonds in a s-trans conformation. B is an s-cis conformation. C has a cis alkene and an s-cis conformation. D has two cis alkenes. E is an isolated diene.
Qu29: D
The key facts here are (1), like alkenes, alkyl groups stabilise alkynes so internal is more stable than terminal and (2) branched chains are more stable than unbranched chains. Therefore the most stable system is D where we have an internal alkyne with the more branched substituents. B, C and E are terminal alkynes. A the substituents are linear not branched.
Qu30: E
Vinyl carbons are carbons that are part of alkenes. A has 2, B has 2, C has 3, D has 2 and E has 4.
Qu31: ACD
sp hybridisation is typical of alkynes and other triple bonds (e.g. nitrile groups) and cumulated systems like allenes.
Qu32: E
This is hydration of the alkene to give an alcohol following Markovnikov's rule, the reaction that is the fastest will be the one that proceeds via the most stable carbocation. A gives a secondary carbocation, B a tertiary carbocation, C and D are slow since they would lead to unfavourable vinyl carbocations and E would give a secondary benzylic carbocation.
Qu33: C
All the bonds are CC bonds, so the triple bond, C will be the shortest.
Qu34: DE
13C peaks in the 190-220 ppm range are carbonyls in aldehydes and ketones. Glycerol (biodiesel by product) is an alcohol, biodiesel is an ester, terephthalic acid is a carboxylic acid, benzophenone is a ketone and benzaldehyde is an aldehyde.