353 Winter 2007 FINAL
Here is an post-mortem analysis / "how to" for this exam. The questions are split by the sections. At the start of each section are a few suggestions of what to look for or how to tackle the question type.
RELATIVE
PROPERTIES:
Identify the controlling feature, which is not always as obvious as it may appear.
Look for two pairs of similar systems to compare that have minimal differences
in structure. If a compound is named, draw it out. If a reaction is involved,
identify the type of reaction and then what the controlling factors are.
Qu1: A
The reactivity of carbonyl groups towards a hydride reagent, looking at the
relative reactivity of i aldehyde,
ii aldehyde,
and iii amide.
Electronic and steric factors need to be considered. First compare ii and iii, aldehydes
tend to be more reactive than amides because (1) they are less hindered
and (2) amine groups are strong electron donors. Both factors make the carbonyl
C less electrophilic and more hindered hence less reactive. The reactivity
of carbonyl systems is impacted by the substituents attached to the carbonyl,
here that is -H and -NR2. The stronger that group is as an electron donor, then
the less electrophilic the carbonyl carbon is. Now what about the two aldehydes
? The 3 Cl atoms in i withdraw electron density via inductive effects
due to their electronegativity making the chloral system, i, more reactive
than a simple aldehyde like ii. Hence in terms of reactivity i
> ii > iii.
Qu2:
AB
The reaction is electrophilic
aromatic substitution, a halogenation,
and we need to look at the substituent
effects on the aromatic ring. The amide group in i is connected via the carbonyl group and so
is a strong electron withdrawing group via resonance - it's a deactivating group.
The chloro group in ii is a weakly deactivating group due to an
inductive effect. The amide group in iii
is attached to the ring via the N attached by single bonds (i.e. the amine N)
of this group. This N has then lone pairs that can be donated to the ring
and hence it is an electron donating group and hence is an activator.
So the reactivity is iii > ii > i.
Qu3:
B
Acidity...
if you know your pKa's then this is easy : carboxylic
acid = 5, aldehyde
enolate = 17 and dicarbonyl
/ active methylenene = 11. Remember the lower the pKa the stronger
the acid, so i > iii > ii.
What if you don't remember your pKas ? (why not ?) Then you'll need
to deduce it. Think
of the simple acid equation HA <=> H+ A-
then look for factors that stabilise A-.....Note that all the acidic H are alpha
to at least one carbonyl group. Now look at the atom the H is attached to.
In ii and iii it's C and i it's O. Recall, that C is less electronegative
than O so C is less stable as an anion compared to O, hence the carboxylic acid
is more acidic than the aldehyde. In the active methylene, the enolate
can be resonance to two C=O groups making it more acidic than a simple aldehyde
like ii.
Qu4:
E
The easiest way to answer this, is by knowing that the rate of catalytic hydrogenation
(e.g. H2 / Pd) is dictated by the pi bond strength and that alkynes
reduce faster than alkenes and alkenes reduce faster than carbonyls, so in terms
of pi bond strength, iii > i > ii. Note : The pi
bonds in an alkyne are on average weaker than those in alkenes (in kcal/mol
the CC bond strengths are for single / double / triple = 88 / 146 / 200 so alkene
pi bond = 146 - 88 = 58, alkyne pi = 0.5 * (200 - 88) = 56. In the carbonyl
group in iii, the C=O = 179 vs C-O = 91 so the pi bond = 88.
Qu5:
C
The systems are substituted alkenes.... electropilic
addition of HCl to an alkene.... this is governed by the stability of the
carbocation produced. If you look at the three alkenes, the key change is that
there is a methyl substitutent in i, a methoxy group in ii and
a acyl group as part of an ester in iii. So
i will give a simple secondary carbocation. In ii and iii
the substituents will impact the stability of the cation. An alkoxy group, RO-
is able to stabilise the +ve charge by resonance so ii will be more reactive
than i (think of the electron donating alkoxy group making the C=C more
electron rich). In contrast, the -CO2R group is electron withdrawing
and will hence destabilise an adjacent +ve charge making iii less reactive
than i ... so ii > i > iii.
Qu6:
A
The N is the common theme, and a nitrogen atom with a lone pair is a potential
base. First, note that the N in iii has four groups attached and hence
has a formal positive charge - there is no lone pair so the N there is not basic.
i and ii are both aromatic
systems. In pyridine i, the lone pair is in an sp2 hybrid and is
not part of the pi system. So protonation of pyridine gives an aromatic conjugate
acid. However, in contrast, in pyrrole ii, the lone pair is in a p orbital
as part of the aromatic sextet. So protonation of pyrrole on N would create
a non-aromatic conjugate acid - this loss of aromatic stability means that the
pyrrole N is a lot less basic than the pyridine N. In terms of pKa pyridine
= 5.2, pyrrole = -3.8. Therefore in terms of basicity, i > ii
> iii.
Qu7:
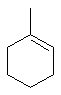
A
Draw out 1-methylcyclohexene... the reaction is the oxymercuration
/ demercuration of an alkene. This gives the Markovnikov alcohol without
any rearrangement (no C+ intermediate) as the major product that is i.
The anti-Markovnikov product would be ii and some of this would also
be formed. Product iii can not be formed from this alkene under these reaction
conditions, so the yields of i > ii > iii.

Qu8:
D
The resonance energy of a polyene increases as the conjugation increases and
if the system is aromatic (aromaticity
is a major effect). i is a non-conjugated diene, this means it has no
resonance stabilisation. ii is benzene, an aromatic "triene"
and iii is a conjugated triene. Therefore ii has the greatest resonance
stabilisation, so ii > iii > i.
Qu9:
A
Propanone is a ketone - they react via nucleophilic
addition with nucleophiles.... so i - iii are the nucleophiles.
i is a Grignard
reagent and should be viewed as a carbanion - since C is not very electronegative,
this is a very reactive nucleophile. If we compare ii and iii
we have S vs O... both from the same group of the periodic table... so from
Chem 351 we should know that the larger S atom is the better nucleophile. Hence
i > ii > iii.
Qu10:
A
A question about carbocation
stability. i is a tertiary cation plus it has allylic
resonance stabilisation. Ii is a tertiary cation and iii is
the phenyl cation which is between primary and methyl (no resonance delocalisation
of the cation here - wrong orbital geometry). Therefore is terms of stability,
i > ii > iii.
LABORATORY:
Based on the general principles cover in the laboratory so far. Need to know
the principles and details of the steps in the experiments.
Qu11:
Qu12:
E
Boiling points need to be corrected to the sea level value - the sea level value
is higher than the altitude value. The rough rule of thumb is about 1 degree
for every 15 C above 50C. So a boiling point of 288 C is 230 C above 50 C and
that means 230/15 correction = 15.8 C. So the corrected value is of the order
of 16C therefore 288+16 = 304 C.
Qu13:
E
From the spectra, the unknown contains a C=O (13C NMR) and aromatic C and H
(both NMRs). There does not appear to be any -OH or -NH (not in H-NMR). So it
is not an acid, alcohol or amine and therefore should only dissolve in an organic
solvent. In fact the structure is benzophenone :

Qu14:
ACDE
Since the unknown is benzophenone (see above) the only positive test would be
the 2-4-DNP test for aldehydes and ketones. A detects alkenes or alkynes, C
is for phenols, D is for methyl ketones and E is for aliphatic alcohols.
Qu15:
C
Know your functional groups from lectures or from the unknowns experiment....
Qu16:
D
The 13C-NMR shows a carbonyl in the aldehyde / ketone range (190-220ppm).
Tollen's test is for aldehydes, so if it is not an aldehyde it must be a ketone
Qu17: ABDE
Recall the procedure used in the aldol experiment and the questions in the
laboratory report. The
one that is obviously wrong is C since enolates are negatively charged and hence
are nucleophiles.
Qu18: B
Draw out 3-pentanone and work through the aldol condensation to get the
product.

STRUCTURES and PROPERTIES:
You need to know about functional groups and reactions, and how to apply concepts
related to structure such as hybridisation, aromaticity
acidity, and reactivity.
Qu19: A
Assign
the configurations as R or S at the chriality
centers.... note that since C and E have easily seen vertical mirror planes
then they can't be (R,R) or (S,S), they must be (R,S) (i.e. they are meso compounds).
The group priority order is Br > CHBrEt > Et > H. B and D are (R,R).
Qu20:
AB or AD
If you have the configurations from qu 19 worked out, then select the systems
with the opposite configurations at all the chirality centers.
Qu21:
BD
Conformational isomers can be interconverted by rotations about single bonds.
That means that they have the same configurations (i.e. R or S). C and E are
different views of the same structure so they are not different conformations.
Qu22:
ABD
Cis-hex-3-ene
will react with bromine via an anti addition to give (R,R)- and (S,S)-3,4-dibromohexene.
Qu23:
C
The systems are all carbonyls, some are mono-, the others are dicarbonyls -
since these are 1,3-dicarbonyls, they are active
methylene compounds, where the middle -CH2- can be deprotonated
to give an anion that can be delocalised by resonance to two different carbonyl
groups. These active methylenes are more acidic than simple aldehyde or ketone
enolates (A and B) and the 1,3-diketone C is the most acidic (see
pKas).
Qu24: C
A has 3, B had 6, C has 8, D has 5 and E has 2. Enolisable
H are those H alpha to a carbonyl group.
Qu25:
E
Carbonyls are reduced by lithium aluminium hydride to alcohols. A gives ethanol,
B gives 2-propanol, C gives 2,4-pentanediol, D gives 1,3-butanediol.
Qu26:
CDE
The systems are all carbonyls, some are
mono-, the others are dicarbonyls - since these are 1,3-dicarbonyls, they are
active
methylene compounds, where the middle -CH2- can be deprotonated
to give an anion that can be delocalised by resonance to two different carbonyl
groups.
Qu27:
AB
If n=2 in the Huckel
rule, we have a 10 pi electron system. C and E are 6 pi aromatic systems,
D is non-aromatic.
Qu28:
D
All the others are aromatic,
A and B are 10 pi, C and E are 6 pi.
Qu29:
C
Only B, C and E are heterocycles. N is less electronegative than O so it would
be more willing to donate its electrons, hence N is more basic. In B the lone pair is part of the aromatic pi system while in C, the N is not part of the aromatic pi system and is therefore more available (more basic).
PRODUCTS
OF SYNTHESIS:
If you are trying to find the product, then you should probably just work forwards
through the sequence of reactions.
Basically depends on the need to know and identify the reactions, this is often
triggered by looking at the functional groups in the molecules.
Qu30:
D
The first step, alkyl halide and base, will convert the phenol
-OH into a methyl ether via a Williamson
type ether synthesis. This is followed by an electrophilic
aromatic substitution, specifically nitration....
this will occur on the most
activated ring which is the -OR substituted ring. -NO2 is deactivating and
-H is the reference (i.e. neither activating or deactivating). Since the -OR
is activating, it is an ortho / para director.
Qu31:
A
The first step is hydride reduction. Since LiAlH4 is a strong reducing
agent it will reduce both the aldehyde
and the ester
to primary alcohols, in this case forming 1,3-propanediol. The diol will then
react with the ethanal, an aldehyde to form a cyclic
acetal.
Qu32:B
Dehydration
of the alcohol forms the alkene, which is then epoxidised
using the peracid. Reaction of the epoxide with the Grignard reagent, a
strong Nu, will occur at the least
substituted end in an SN2 fashion to give the alcohol.
Qu33:D
The cyclic
anhydride is a carboxylic acid derivative and will react with the ammonia
(a Nu) to open the ring and form the amide and carboxylate (push the arrows!)
Qu34:B
The starting material
is really just a tosylate,
so we are looking at a simple SN2
reaction with the tosylate acting as the leaving group to give the primary
bromide.
Qu35:E
The aryl bromide reacts with the magnesium
to form the aryl Grignard reagent which is turn is reacted with the ester
to form a tertiary alcohol.
Qu36:A
The alkene
undergoes ozonolysis with a reductive work-up to give the methyl ketone
/ aldehyde system. This is then followed by a acid catalysed aldol
condensation so the enone feature is a good hint.
STARTING MATERIALS FOR SYNTHESIS:
Need to be able to work backwards....
but again look at the functional groups in the products to think about how
you may have got there.
How well do you know your reagents
? Look at what has actually happened in terms of the reaction functional
group transformation and then first look for any regiochemical issues then
finally the stereochemistry last (it's the hardest to sort out).
Qu37:
A
PCC
is an oxidant, so the ketone has been made from secondary alcohol. The previous
pair of reaction conditions correspond to the oxymercuration
/ demercuration of an alkene, which looks to have been made by a Diels-Alder
reaction. So put the double bond is then push the arrows to reveal the diene
and dienophile. Since we are told the diene is 1,3-butadiene we should be able
to see the dienophile is cyclohexene, A
Qu38: D
Since we are looking at a ketone as the product, the Hg2+/ aq. acid looks like
an alkyne
hydration. The fact that it's a methyl ketone and the presence of teh phenyl
group mean that it must have been a non-conjugated system and hence it must
have been a simple terminal alkyne. Now looking at the first step, the use of
benzyl bromide confirms that we are just looking for 2 other carbons, so we
have a simple SN2
of acetylide, D.
Qu39:
B
The product is a lactone (a cyclic ester) from a tertiary alcohol and a carboxylic
acid: these would have reacted due to the H+. The first step accounts for the
tertiary alcohol (note the two methyl groups have been added). In order to get
a tertiary alcohol and an acid, B must have been used. A would
still contain Br atoms, C would give a secondary alcohol, D would
give 3 tertiary alcohols but not acid group and E would not have either
an alcohol or a carboxylic acid.
Qu40:
C
Vinyl
lithium (CH2=CHLi) is like a Grignard reagent, the C=C from the
vinyl group is clearly visible in the product : those 2 C have been added. The
product is also a ketone. A glance at step 1 suggests we have made the ketone
by the addition of the organometallic
reagent to the nitrile, which is turn would have come via the reaction of
the CuCN
with a diazonium salt, C.
Qu41:
A
Catalytic
hydrogenation .... since the product is a hydrocarbon, it's probably the
reduction of an alkene or alkyne. Step 2 looks like a Diels-Alder
reaction. Therefore is we have the cyclohexane system with 6 C atoms, then
since the butadiene accounts for the source of 4 of those 6 C, the dienophile
accounts for the other 2 and these must have the 2 methyl groups and the ethyl
group and hence be an alkene. For the stereochemistry to work out, the 2 methyl
groups need to be cis in the alkene. Step 1 tells us that the alkene has been
made by the elimination
of an alkyl halide.... therefore the answer must be either A or B. The small
base, HO- and heat implies an E2
and the antiperiplanar requirement implies that A is the required starting
material. B would give the wrong alkene stereochemistry. You may need
to draw A and B in the antiperiplanar geometry to convince yourself.
Qu42:
C
Aq. acid and heat are always hard to work out.....so skip for now....
Step 2 is aromatic
nitration. That explains the nitro group on the benzene. Now since we also
have an amine, it looks like step
1 protects the amine as the amide and step
3 removes the amide group. This also helps direct the reaction para and
changes the properties of the amine N making it less basic (nitration of aniline
is complicated by the acid/base reaction with the strong acids which turns the
aniline into an NH3+ group which is deactivating and meta directing).
Qu43:
B
Aqueous bromine forms a halohydrin
from an alkene via an anti addition. A and B have the correct
substituents. Draw one of the products in a wedge-hash diagram with the Br and
OH in the plane but anti to reveal the stereochemistry of the starting alkene.
REAGENTS FOR SYNTHESIS
How well do you know your reagents ? Look at what has actually happened in terms
of the reaction functional group transformation and then first look for any
regiochemical issues then finally the stereochemistry last (it's the hardest
to sort out).
Qu44:
B
The first steps are alkylation of an active methylene ester enolate, so we need
a base...given that we have ethyl esters, ethoxide is a good choice here.
Qu45:
AE
Now we need the alkylating
agent, looking for an allylic halide.
Qu46:
BE or CD
We need to remove one ester group and hydrolyse the remaining one to a carboxylic
acid.... this looks like hydrolysis and decarboxylation. Aqueous acid or base
and heat are the best for this.
Qu47:
BC
Now reform the ethyl ester... use ethanol, acid catalyst and heat.
Qu48:
D
The conversion is of an aldehyde to a C=C, and we have added 3 more C atoms...
looks like a Wittig reaction so look for the Ph3P system.
Qu49:
A
Formation of a cyclohexene from a diene looks like a Diels-Alder reaction, push
the arrows backwards to reveal the dienophile to look for (Br and CHO groups
needed).
Qu50:
DE
Reduce the aldehyde to the primary alcohol but without reducing the ester....
that means be selective... use NaBH4.
Qu51:
AD
Convert the alcohol to a benzyl ether.... a Williamson ether synthesis... need
a base to make the alkoxide, a better Nu, plus benzyl bromide.
Qu52:
C
Alkene to an epoxide using a peracid.
Qu53:
B or CD
Now convert a halohydrin to an epoxide.... this just needs a base.
Qu54:
ABC
Alkene to a 1,2-diol : here we use the osmium tetroxide route.
Qu55:
BD
Now convert an -OH to a methyl ether... another ether synthesis using base and
methyl iodide.
Qu56:
AC
Conversion of the ester to
a tertiary alcohol with 2 new methyl groups.... look for the methyl organometallic
reagent.... here it's methyl lithium.
Qu57:
D
The reaction is radical
addition of HBr to the alkene which proceeds with anti-Markovnikov selectivity,
this is because the first group to add to the C=C is the Br radical which adds
to give the more stable benzylic radical as the intermediate.
Qu59:
C
The key issue here is that twisting of the N substitutent occurs when the alkyl
groups are larger due to steric effects. The twisting removes the resonance
interaction of the N with the aromatic ring and hence "turns off"
the activating effect of the substituent.
Qu60:
E
The reaction that occurs is a trans-esterification where the ethanol reacts
as a Nu to replace the methyl group with ethyl. In order to hydroyse the ester
to the carboxylic acid, then water should have been used.
Qu61:
A
Boiling points are controlled by intermolecular forces. The presence of electronegative
O atoms in the ether THF cause dipoles to be present that result in dipole-dipole
interactions.
Qu62: C
The reaction is the electrophilic addition
of bromine
to the alkyne. The trans stereochemistry occurs as the result of the intermediate
cyclic bromonium ion that forces the bromide ion in the second step to add from
the opposite side to the Br in the bromonium ion that effectively blocks one
face of the pi system.