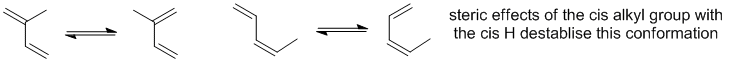
Here is an post-mortem analysis / "how to" for the Final. The questions are split by the sections. At the start of each section are a few suggestions of what to look for or how to tackle the question type.
RELATIVE PROPERTIES:
Identify the controlling feature, which is not
always as obvious as it may appear. Look for two pairs of similar systems to compare
that have minimal differences in structure. If a compound is named, draw it out.
If a reaction is involved, identify the type of reaction and then what the controlling
factors are.
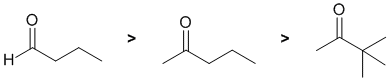
Qu1:
The reactivity of carbonyl groups of an aldehyde and two ketones towards sodium borohydride The electronic and steric factors of each of the substituents on each of the carbonyl groups need to be considered. In the aldehyde we have a neutral H atom (i.e. no electronic effect and small). In the ketones, which are both methyl ketones, the difference is in the other -R group (an alkyl group) where we have n-propyl and t-butyl groups. In general, alkyl groups are weak electron donors but we also need to consider their sterics. Electron donors on the carbonyl make the carbon less electrophilic, larger groups impede the access of the Nu so these factors will make the carbonyl less reactive. Hence in terms of reactivity towards hydride, aldehydes are more reactive than ketones. A more sterically hindered methyl ketone will be less reactive, so, overall we have:

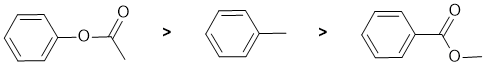
Qu2:
The reaction is electrophilic aromatic substitution, Friedel-Crafts alkylation and we need to look at the substituent effects on the aromatic ring. The substituents are a methyl group, an ester connected via the -O- and an ester connected via the C=O. In the ester that is attached through the C=O is a moderately electron withdrawing group via resonance and induction - it's a moderate deactivating group. For the ester that is attached through the alkoxy O and therefore is a moderate electron donating group via resonance donation of the O lone pair (note that there are competing effects for the interaction of the lone pair towards the arene and the carbonyl groups) - it's a moderately activating group. The methyl groups is a weak electron donor which is weakly activating due to inductive effects. So the reactivity:
Qu3:
The reaction is the Diels-Alder reaction and we are looking at the dieneophiles reacting with the diene (1,3-butadiene). The reactivity increases in the Diels-Alder reaction with electron withdrawing groups on the dienophile. Here we have an alkyl group (weak electron donor), an aldehyde (moderate electron withdrawer) and two ester groups (each moderate electron withdrawer). Therefore :
![]()
Qu4:
The reactivity of C=C pi bonds towards HCl so we are looking at electrophilic addition to an alkene which will be controlled by the stability of the intermediate carbocation (more stable C+ forms faster in the rate determining step). A closer look at the alkene structures shows that each of the alkenes has a different substituent attached: a hydrogen atom, an alkyl group -R, and an alkene C=C. Each alkene will react with the acid to give a carbocation and the substituent will affect the carbocation stability.... an electron donor will stabilise a carbocation, an electron withdrawer will destabilise a carbocation. Looking at the substituents: a hydrogen is electronically neutral, an alkyl group, -R, is a weak electron donor, and an alkene is an electron donor through resonance. So in terms of reactivity of the alkenes towards H+ :
![]()
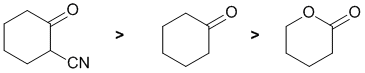
Qu5:
Acidity...Remember the lower the pKa the stronger the acid, and think of the simple acid equation HA <=> H+ A- then look for factors that stabilise A-.... Here we are looking at carbonyl systems and the formation of enolates by the removal of H from the C atom adjacent to the C=O (known as the alpha position) since this allows for resonance stabilisation of the -ve charge. In a simple ketone, such as cyclohexanone, the pKa is about 20, in an ester the pKa is about 25. Finally, the ketone with the alpha-nitrile... a nitrile is also an electron withdrawing group so this is really an example of an active methylene and hence it will be more acidic than a simple ketone. Therefore, in terms of acidity:
Qu6:
This question relates to
resonance energy and aromaticity. Aromatic systems have high resonance stablisation and therefore high resonance energies. Conjugated systems also have resonance stabilisation but not to the same extent as aromatics. These 3 systems were discussed when we discussed how to evaluate resonance energy and the aromaticity of benzene (see the figure in the link above). Here we have three trienes, one isolated and two conjugated, but one is also aromatic. Hence, it terms of resonance energies:
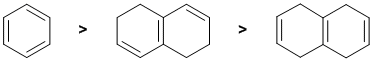
Qu7:
Oxidation states...The easiest way to reach a partial answer is based on the knowledge that aldehydes can be reduced to primary alcohols. More bonds to O (or more electronegative atoms) typically means a higher oxidation state. More formally, we count the bonds attached to the atom being considered. A bond to a more electronegative atoms (e.g. C attached to O or N) counts -1, a bond to the same type of atom counts 0 and a bond to a less electronegative atom (e.g. H) counts +1. Total the count and then consider the formal charge on the central atom since the oxidation state for the central atom plus the groups attached must equal the atoms formal charge. For the nitrile, the C is attached to 3 x N (count - 1 each, total -3) and 1 x C (count 0) therefore total = -3 and therefore the oxidation state C = +3. In the aldehyde, the C is attached to 2 x O (count - 1 each, total -2) and 1 x C (count 0) and 1 x H (count +1) therefore total = -1 and therefore the oxidation state C = +1. In the primary alcohol, the C is attached to 1 x O (count -1), 1 x C (count 0) and 2 x H (count +1, total +2) therefore total = +1 and therefore the oxidation state C = -1.
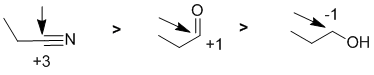
Qu8:
The reaction is the hydroboration / oxidation of an alkyne. The issue is the regioselectivity. The alkynes are all internal and all are 2-alkynes (i.e. a methyl group at one end) and a different group at the other end : methyl, ethyl and isopropyl. One should also note that one of the alkynes is symmetrical and the other 2 are not. Recall that the regioselectivity of hydroboration is affected by steric effects. A methyl ketone will be the product if the B adds to at C2 of these alkynes. Hydroboration / oxidation of but-2-yne (symmetrical) doesn't have regiochemistry to worry about, the product will be butan-2-one. Of the other 2 alkynes, adding the B to C2 will be more likely the larger the group (isopropyl > ethyl) at C3. Hence:
![]()
Qu9:
The reaction is electrophilic aromatic substitution, bromination and we need to look at the substituent effects on the aromatic ring. The substituents are a t-butyl group, a chlorine and a trichloromethyl. The t-butyl group (-C(CH3)3) is a weak electron donating group and therefore directs o,p. Due to steric effects at the adjacent ortho (o) position, the major product will be para (p). The chlorine is a weakly electron withdrawing group but still directs o,p but without much of a steric effect so a reasonable amount of o will also form. The trichloromethyl group is an electron withdrawing group and directs meta (m). So % para:
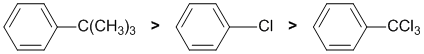
Qu10:
All about absolute configuration and optical rotation. Assign the configurations using the Cahn-Ingold-Prelog priority rules, and remember that (1) enantiomers have specific rotations of equal magnitude but opposite sign and (2) that meso compounds are optically inactive (zero rotation).
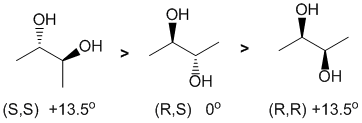
STRUCTURES and PROPERTIES:
You need to know about functional groups and reactions, and how to apply concepts related to structure such as stereochemistry, acidity/basicity and reactivity etc.
Qu11:
If a molecule does not have a chirality center, it will be optically inactive. The most common scenario for a chirality center in an organic molecule is a C atom with 4 different groups attached. The chirality centers are marked with *...
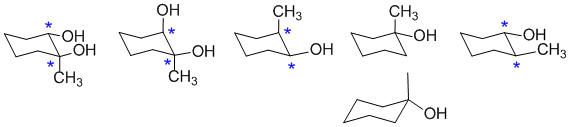
Qu12:
Hydroboration / oxidation of 1-methylcyclohexene will give the anti-Markovnikov alcohol (i.e. -OH at the less substituted position) via a syn addition (i.e. -OH and -H are added to the same face of the alkene and thus the -OH and -CH3 end up trans). Note that the enantiomer will also be formed, but it is not one of the answer options:
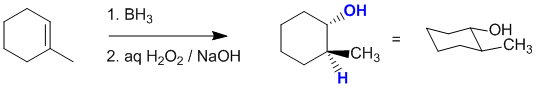
Qu13:
Acid catalysed hydration of 1-methylcyclohexene will give the Markovnikov alcohol (i.e. -OH at the more substituted position) via the planar carbocation:
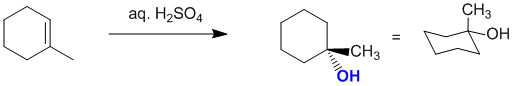
Qu14:
The peracid will react with 1-methylcyclohexene to give an epoxide. The epoxide will then react with aq. NaOH to give a 1,2-trans diol: Note that the enantiomer will also be formed, but it is not one of the answer options:
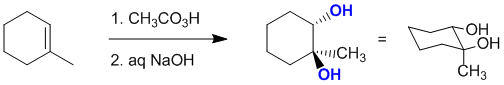
Qu15:
Cold alkaline permanganate will react with 1-methylcyclohexene to give a 1,2-diol via a syn addition: Note that the enantiomer will also be formed, but it is not one of the answer options:
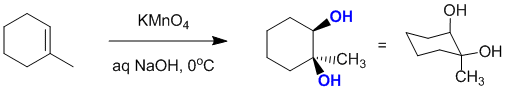
Qu16:
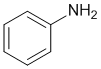
Look at the substituent effects on the aromatic ring... -NH2 groups are strongly electron donating due to the lone pairs on the N atom adjacent to the ring (via resonance) and N is less electronegative than O, so aniline is the most activated of these benzene systems.
Qu17:
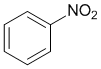
Look at the substituent effects on the aromatic ring... -NO2 groups are strongly electron withdrawing (induction and resonance) so nitrobenzene is the most deactivated of these benzene systems.
Qu18:
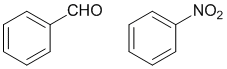
Look at the substituent effects on the aromatic ring... electron withdrawing groups are deactivating and direct meta and are characterised by the atom attached to the ring being formally or partially +ve as a result of several bonds to more electronegative atoms. Note that while halogens such as -Cl are deactivating, they direct ortho and para.
Qu19:
Friedel-Crafts acylations only work on benzenes that are as active as monohalobenzenes or better so the halo-, alkyl- and alkoxy- benzenes... Aminobenzenes also fail due to the nucleophilicity of the N atom.
AROMATICITY and RESONANCE:
Best method is to work through the molecules and decide on the aromaticity based on the four criteria for the pi system (cyclic, planar, conjugated pi system with 4n+2 pi electrons).
Qu20:
To be non-aromatic as drawn, we need to find a system that fails on one of the first three criteria (i.e. it lacks a cyclic conjugated pi system) but has a conjugate base that satisfies all four of the criteria for aromaticity (i.e. it has a pi system that is cyclic, planar, fully conjugated and contains 4n+2 pi electrons). The most likely scenario will involve loss of H+ from a sp3 center to create a conjugated lone pair (so adding 2 electrons to the pi system) ... for clarity the aromatic resonance structures of the conjugate bases are also shown:
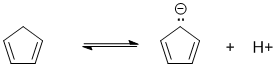
Qu21:
To be non-aromatic as drawn, we need to find a system that fails on one of the first three criteria (i.e. it lacks a cyclic conjugated pi system) but has a conjugate base that satisfies the first 3 of the criteria for aromaticity (i.e. it has a pi system that is cyclic, planar, fully conjugated) but then contains 4n pi electrons). The most likely scenario will involve loss of H+ from a sp3 center to create a conjugated lone pair (so adding 2 electrons to the pi system):
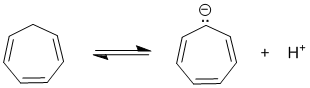
Qu22:
A diene has two C=C and they need to be isolated in order to have no resonance energy stabilization.
Qu23:
In order to have the highest "n" in the Huckel rule (4n+2 pi electrons) then we need to most C=C units.
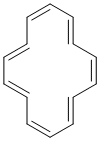
Qu24:
To be aromatic and a triene, we need to find a system that satisfies all four of the criteria for aromaticity (i.e. it has a pi system that is cyclic, planar, fully conjugated and contains 4n+2 pi electrons) and has just 3 C=C.
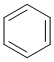
Qu25:
The most acidic H are likely to on the cationic systems (where the conjugate base will be neutral) related to pyridine and pyrrole. The pyrrole conjugate acid is the more acidic because while the conjugate acid is non-aromatic, pyrrole itself is aromatic so there is a significant extra stabilisation of the deprotonated form. For pyridine, both the conjugate acid and pyridine are both aromatic.
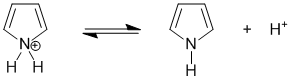
Qu26:
The availability of the electrons is the key factor.... hybridisation of the orbital containing the lone pair and resonance are key factors. Lone pairs in sp3 orbitals are further from the positive nucleus and are more available and more basic.
Qu27:
The most likely to donate a hydride will be a non-aromatic system that can lose H- to give an aromatic cation:
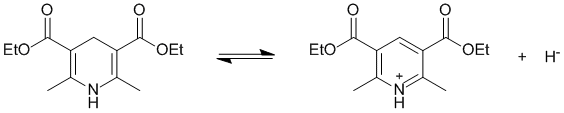
Qu28:
To be non-aromatic as drawn, we need to find a pi system that fails on one of the first three criteria for aromaticity (i.e. it lacks a cyclic conjugated pi system) but has a resonance contributor that satisfies all four of the criteria for aromaticity (i.e. it has a pi system that is cyclic, planar, fully conjugated and contains 4n+2 pi electrons). The most likely scenario will involve an exocyclic double bond unit:
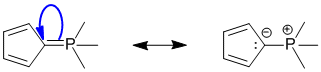
Qu29:
To be an antiaromatic, compound we need to find a structure that has a pi system that follows the first three criteria for aromaticity but is a 4n system in the Huckel rule (i.e. an even number of pi electron pairs). This structure has 2 x C=C ( 2 pi pairs) and a cyclic conjugated system by virtue of the empty p orbital at the B atom (check the periodic table).
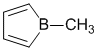
STARTING MATERIALS AND PRODUCTS:
If you are trying to find the product, then you should probably just work forwards through the sequence of reactions.
If you are looking for the starting material, then working backwards is probably the best way to go....
Basically depends on the need to know and identify the reactions, this is often triggered by looking at the functional groups in the molecules.
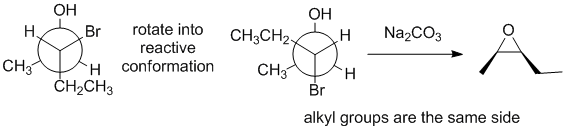
Qu30:
Working forwards : the halohydrin reacting with Na2CO3 (a weak base) to make an epoxide via an SN2 reaction so we need to make sure we view the halohydrin in the reactive conformation with the O nucleophile undergoing a backside attack to the C-Cl bond.
Qu31:
Working backwards.... the product is a carboxylic acid which looks to have been made by the oxidation of an alkyl group (with a benzylic H) on the benzene ring. The first step is a Friedel-Crafts alkylation that gave a para- (or 1,4-) product : which means we need an activating group that directs o,p e.g. an alkyl group. Since tert-butyl groups don't get oxidised, we must have had ethylbenzene to start with:
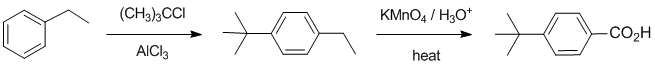
Qu32:
Working backwards....the product is a tertiary alcohol which has been formed using a Grignard reagent (ethyl magnesium bromide) and therefore was obtained from the ketone propan-2-one (aka acetone). Ketones can by made by the hydration of alkynes (count C atoms), while the hydration of alkenes gives alcohols.
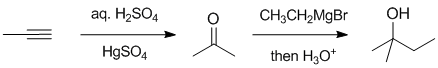
Qu33:
Working forwards....Friedel-Crafts acylation of benzene gives the aromatic ketone. Clemmensen (Zn-Hg / HCl) reduction converts the C=O to a CH2 which then undergoes bromination via electrophilic aromatic substitution at the para position:
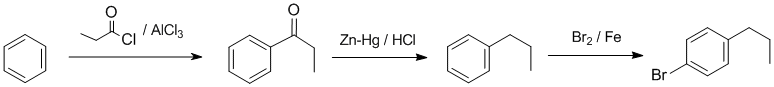
Qu34:
Working forwards.... The conjugated diene undergoes a Diels-Alder reaction with maleic anhydride to give the bicyclic product. Reaction of a cyclic anhydride with methanol will give an ester and a carboxylic acid.
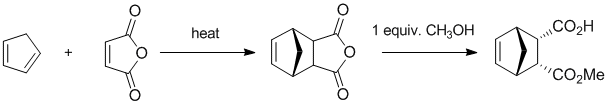
Qu35:
Working forwards.... Dissolving metal reduction of the internal alkyne gives the trans-alkene. Reaction with the peracid provides the trans-epoxide with the then converted to the 1,2-diol with aq. acid (equivalent to anti addition to the alkene). The 1,2-diol reacts with the ketone to form a cyclic ketal:
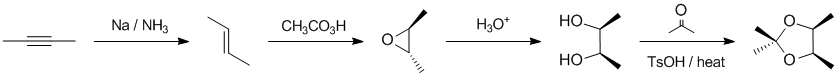
Qu36:
Working forwards....The amide is reduced to the amine with LiAlH4 (count C atoms) but then reaction of the amine with the acid chloride generates a new amide:
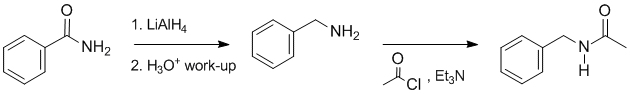
Qu37:
Working backwards.... The product is a cyclic, conjugated aldehyde made with base / heat, all signs of an intramolecular aldol of a dicarbonyl from via ozonolysis... count C atoms !
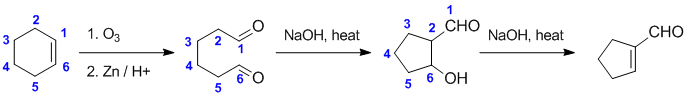
Qu38:
Working forwards.... the hydration of alkynes will give a methyl ketone, that then underwent a Baeyer-Villager reaction of a ketone to give an ester. The "O" is inserted on the more substituted sid to give the t-butyl ester.
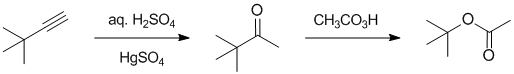
REAGENTS FOR SYNTHESIS:
Need to be able to look at reactions, looking at the functional groups in the starting materials and products of each step to think about
how you have got there.
How well do you know your reagents
? Look at what has actually happened in terms of the reaction functional
group transformation and then first look for any regiochemical issues
then finally the stereochemistry last (it's the hardest to sort out).
Qu39:
The product has 2 more C atoms from a new ethyl group and is a 1,2-dibromide. The starting material is a terminal alkyne. Need to alkylate the terminal alkyne adding two carbon atoms using NaNH2 then ethyl bromide. Now for the stereochemistry... the product we require is not a meso compound. Since the addition of Br2 to alkenes proceeds via an anti addition, to get the right product stereochemistry, the alkene must have been cis and therefore obtained from the alkyne via a partial catalytic reduction using H2 / Lindlar's catalyst. So : (1) NaNH2 then CH3CH2Br, (2) H2 / Lindlar's catalyst, (3) Br2.
Qu40:
The product has 1 more C atom than the starting material. Hydration of the terminal alkyne using aq acid with Hg salt catalyst will give a methyl ketone. The ketone can participate on a Wittig reaction with Ph3PCH3 / NaH (which generates the ylide) to give the required alkene. Therefore : Hg(OAc)2, H3O+, (2) Ph3PCH3 / NaH.
Qu41:
Comparing the product to the starting material, the alkene has been replaced with an extra methyl group and an alkoxy group with specific stereochemistry.
Grignard reagents don't react with simple C=C systems, but they do react with epoxides that leave a O containing functional group for further elaboration.... so convert the alkene to the epoxide using a peracid (e.g. mCPBA) , then treat with the Grignard reagent MeMgBr after the work up (which gives an alcohol), the alcohol is converted to the ether using NaH (to form an alkoxide as a nucleophile) then MeI : (1) mCPBA, (2) MeMgBr then H3O+, (3) MeI / NaH.
Qu42:
The product is a disubstituted benzene : an alkyl ammonium benzene. Therefore, Friedel-Crafts alkylation followed by nitration and then reduction is required : (1) MeCl / AlCl3 (2) H2SO4 / HNO3 (3) Sn / HCl.
Qu43:
Reduction of an amide to an amine but without reacting the ketone will require protection of the ketone (as a ketal) before the reduction and finally removal of the protecting group : (1) HOCH2CH2OH, TsOH, H3O+, (2) LiAlH4 / THF then H3O+, (3) H3O+, heat.
Qu44:
C atoms have been lost, and an O functional group (phenyl ether) obtained via a Baeyer-Villager reaction of a ketone to give an ester, followed by ester hydrolysis and then ether synthesis : (1) mCPBA, (2) NaOMe, MeOH, heat, (3) LDA (a base) and MeI.
EXPLANATION OF PHENOMENA
Qu45:
When ranking basicity, looking at the availability of the lone pair electrons is the best approach. For N1, the pyridine N, the lone pair is in an sp2 hybrid orbital that is perpendicular to the aromatic pi system. For N2, the amino group N, the lone pair is in a p orbital that is involved in a resonance interaction with the aromatic pi system. Therefore, N1 lone pair electrons are more available than those on N2 and so N1 is more basic than N2. This means that on protonation of N1 to give it's conjugate acid, the amine group can stabilise the positive charge due to its electron donating character.
Qu46:
The reaction is the hydride reduction of a ketone to a secondary alcohol where the hydride is the nucleophile and attacks the C=O from the less hindered side.
Qu47:
It's the group on the ring (-Br) that directs the substitution, halogens are deactivators but direct ortho, para. The size of the initial substituent blocks the adjacent ortho positions leading to an increase in the yield of the para product.
Qu48:
The amide is more acidic of this pair. In the more acidic amide (pKa about 15), there is at least one H attached to the N atom (remember that N is electronegative and can stabilise the conjugate base). In the ester, the O has only C atoms attached and the most acidic H is alpha to the carbonyl group attached to a less electronegative C atom (pKa about 25).
Qu49:
The reaction is a Diels-Alder reaction. The more reactive diene will be the one that can most readily achieve the reactive s-cis conformation.